Abstract
Iatrogenic aortic dissection occurs in association with diverse invasive procedures. It occurs primarily as a complication of cardiac surgery or after catheterization procedure. We found a case of iatrogenic abdominal aortic dissection caused by traumatic needle injury. The patient complained of abdominal pain after balloon kyphoplasty. Abdominal computed tomography (CT) showed intramural hematoma with air density suggestive of an iatrogenic cause. The patient was managed conservatively, and no lesion progression was noted in the follow-up CT. To the best of our knowledge, this is the first case of iatrogenic aortic dissection associated with kyphoplasty.
Iatrogenic aortic dissection (AD) is a rare complication associated with various invasive procedures. Because classic symptoms for iatrogenic AD are often absent, a clinical suspicion is a key to diagnosis. Though many cases of iatrogenic AD develop in association with an endovascular procedure, it may also occur as a result of traumatic needle injury. Balloon kyphoplasty includes an initial process that involves a guiding pin, during which traumatic aortic injury may be develop. In this report, we present the first case of iatrogenic AD after kyphoplasty.
A 79-year-old woman visited the emergency department complaining of aggravating abdominal pain. She had been diagnosed with hypertension and diabetes mellitus 5 years ago. Four months ago, she had undergone percutaneous balloon kyphoplasty for T12 vertebral compression fracture. Her back pain was alleviated, but she complained of gradually escalating abdominal pain after the procedure.
On admission, her blood pressure was 110/70 mmHg, pulse rate 78 beats per minute, temperature 36.6℃, and respiratory rate 20 breaths per minute. Physical examination showed tenderness on palpation in the right upper quadrants.
The laboratory findings were as follows: white blood cell count was 12,100/µL, with a neutrophil count of 75.7%; hemoglobin level was 12.1 g/dL; platelet count was 265,000/µL; aspartate transaminase level was 16 IU/L; alanine transaminase level was 11 IU/L; alkaline phosphatase level was 58 IU/L; total bilirubin level was 0.8 mg/dL; and D-dimer level was 3.39 mg/L. The levels of cardiac markers were within the normal limits. An electrocardiogram showed normal sinus rhythm with a heart rate of 76 beats per minute. No signs of ischemia were observed.
Chest and abdominal radiography findings were unremarkable. Echocardiography showed that the ejection fraction of the left ventricle was 49.8%; regional wall motion abnormalities, pericardial effusions, or pleural effusions were not observed.
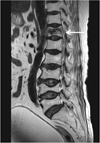
Abdominal computed tomography (CT) showed that the gallbladder and bile duct were normal. However, since there was suspicion of pulmonary emboli in the right pulmonary arteries and abdominal AD, an additional angiographic CT was performed. This showed pulmonary thromboembolism in the right main, lobar, and segmental pulmonary arteries, with deep-vein thrombosis in the left distal femoral vein (Fig. 1). Furthermore, proximal abdominal AD was observed from the level of the celiac trunk to that of the left renal artery, with thrombus formation in the pseudolumen (Fig. 2a). A small air bubble was observed in the thrombus, and the dissection site was contiguous with the T12 vertebra, where kyphoplasty had been performed (Fig. 2b and 2c). Therefore, we reviewed the magnetic resonance imaging examination before kyphoplasty, which revealed no dissection in the abdominal aorta (Fig. 3).
We considered anticoagulation therapy for the pulmonary embolism, but it was contraindicated because of the AD. Treatment was initiated with the insertion of an inferior vena cava filter and administration of a beta-blocker. Her vital signs were stable, and a follow-up abdominal CT after 2 weeks in hospital showed no interval change in the size of the AD. Her symptoms improved and she was transferred to another hospital in her hometown.
AD is an uncommon condition. Meszaros et al. reported that the incidence of AD was 2.9 per 100,000 population per year.1 AD is defined as iatrogenic when it occurs as a complication of various invasive procedures. According to a study by Januzzi et al., 5% of all AD cases are iatrogenic.23 Most of the patients (76%) with iatrogenic AD belong to Stanford type A. Type A iatrogenic AD is mainly associated with cardiac surgical procedures, whereas type B is mostly related to cardiac catheterization.
This present case occurred as a result of traumatic needle injury during kyphoplasty, not an endovascular procedure. In a comprehensive meta-analysis, Taylor et al. summarized all of the published kyphoplasty complications.4 Cement leakages occurred in 8.1%, new vertebral fractures in 11.1%, spinal stenosis with spinal cord compression in 0.16%, radiculopathy in 0.17%, and pulmonary embolism in 0.17% of all cases. However, none of the patients presented with AD as a complication of kyphoplasty.
Yohan et al., Steven et al. and Lieberman et al. explained kyphoplasty in detail.567 The bilateral transpedicular approach is the standard kyphoplasty ascess for the thoracolumbar spine. Using biplanar fluoroscopy, biopsy needles (mainly Jamshidi needle) are used to enter the fractured vertebra on both sides through the pedicle. Then a stylet and cannula are placed over the biopsy needle into the pedicle. After the biopsy needle and stylet are removed, a hand-mounted drill bit is then used to create a pathway for the passage of the inflatable bone tamp (IBT). The IBT is positioned under the collapsed endplate. Next, the balloon is carefully inflated in an attempt to restore the vertebral body's height. The cavity is then filled with bone cement under low pressure. In the case of our patient, the aorta might have been injured during the initial use of the biopsy needle or stylet. An air bubble might have traveled into the aorta during the balloon inflation or the cement augmentation.
A case of ultimately fatal AD during central venous catheter insertion was reported by McDaniel et al.8 Tsukashita et al. reported a similar case, but the patient had emergent surgery and her long-term outcome was favorable.9 When transaortic insertion of a needle or catheter is suspected, it must be left in place and imaging should be performed to identify the precise injury site within the arterial system.
Januzzi et al. found that iatrogenic AD was more common than spontaneous AD in the older population.3 Patients with iatrogenic AD, as opposed to spontaneous AD, did not present with chest or back pain (1% vs. 25%; p<0.001); furthermore, patients with iatrogenic AD were more likely to show indolent hemodynamic instability than were those with spontaneous AD. Hence, for an rapid diagnosis of iatrogenic AD, a high index of clinical suspicion is required.
To our knowledge, this is the first case of iatrogenic AD associated with kyphoplasty. Even though the signs and symptoms are often atypical, clinicians should be aware of this rare complication. Unexpected chest or abdominal symptoms following the procedure may require imaging study. An air bubble in the thrombus is a very significant sign, suggestive of an iatrogenic cause in patients with AD. The history of the procedures performed in the patient is crucial to diagnosis.
Figures and Tables
Fig. 1
Angiographic CT showed pulmonary thromboembolism in the right main, lobar pulmonary artery (white arrow).
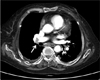
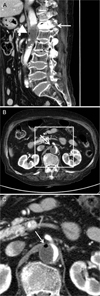
Fig. 2
(A) Enhanced abdominal computed tomography in the sagittal plane performed in the emergency room showed cement masses within the T12 vertebral body (white arrow). Aortic dissection with thrombus formation in the pseudolumen at the T12-L2 vertebral level was observed. An air bubble can be seen in the thrombus (white arrowhead), (B) Axial view at the level of the air bubble(white arrow), (C) A zoomed-in photograph of the air bubble in the thrombus (white arrow).
References
1. Mészáros I, Mórocz J, Szlávi J, Schmidt J, Tornóci L, Nagy L, et al. Epidemiology and clinicopathology of aortic dissection. Chest. 2000; 117:1271–1278.

2. Hagan PG, Nienaber CA, Isselbacher EM, Bruckman D, Karavite DJ, Russman PL, et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA. 2000; 283:897–903.

3. Januzzi JL, Sabatine MS, Eagle KA, Evangelista A, Bruckman D, Fattori R, et al. International Registry of Aortic Dissection Investigators. Iatrogenic aortic dissection. Am J Cardiol. 2002; 89:623–626.
4. Taylor RS, Fritzell P, Taylor RJ. Balloon kyphoplasty in the management of vertebral compression fractures: an updated systematic review and meta-analysis. Eur Spine J. 2007; 16:1085–1100.

5. Garfin SR, Yuan HA, Reiley MA. New technologies in spine: kyphoplasty and vertebroplasty for the treatment of painful osteoporotic compression fractures. Spine (Phila Pa 1976). 2001; 26:1511–1515.
6. Lieberman IH, Dudeney S, Reinhardt MK, Bell G. Initial outcome and efficacy of "kyphoplasty" in the treatment of painful osteoporotic vertebral compression fractures. Spine (Phila Pa 1976). 2001; 26:1631–1638.

7. Robinson Y, Heyde CE, Försth P, Olerud C. Kyphoplasty in osteoporotic vertebral compression fractures--guidelines and technical considerations. J Orthop Surg Res. 2011; 6:43.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download