Abstract
Myocardial bridging (MB) occurs when the myocardium covers a segment of a major epicardial coronary artery, resulting in a tunneled arterial segment. Although MB is generally considered benign, it has been associated with myocardial ischemia. A 70-year-old man with MB (50% luminal narrowing during systole) at the mid-left anterior descending artery (LAD) on previous coronary angiography (CAG) visited our hospital with worsening chest pain. His blood pressure (BP) was not well controlled because of poor compliance. Follow-up CAG showed that MB at the mid-LAD progressed to severe stenosis (>90% luminal narrowing during systole) and the total length of tunneled artery extended from 22.5 to 23.9 mm. His chest pain was relieved by BP control. This is the first report of myocardial ischemia secondary to progression of MB demonstrated by CAG in Korea.
The major epicardial coronary arteries and branches generally run on the surface of the myocardium. Occasionally, part of a coronary artery enters the myocardium and can be compressed during systole. This is called myocardial bridging (MB) and was first reported in 1951.1 Although MB is generally considered benign, it can lead to myocardial ischemia.2 A recent analysis of intracoronary ultrasonography and doppler evaluation showed that relaxation of coronary artery is delayed during early diastole in a coronary vessel with MB, which can decrease the coronary vasodilator reserve.3 Furthermore, progression of left ventricular hypertrophy, increased myocardial contractility and tachycardia can make the tunneled segment deeper and systolic compression aggravated, which can contribute to myocardial ischemia.34 Therefore, MB should be considered a possible cause of myocardial ischemia in a patient with no atherosclerotic coronary artery disease.
However, there are few reports about the change of severity of MB, which is also closely related to the development of myocardial ischemia. We herein report a case in which aggravation of MB in the mid-left anterior descending artery (LAD) was demonstrated by coronary angiography (CAG) and caused myocardial ischemia.
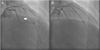
A 70-year-old man visited the outpatient department with recently worsening chest pain. He had been treated for hypertension and diabetes mellitus for 20 years. In 2003, he had been admitted to the hospital for chest pain and underwent CAG, which revealed >80% stenosis of the proximal LAD and MB at the mid-LAD, which showed moderate stenosis (50% luminal narrowing) during systole. He underwent percutaneous coronary intervention for the severe stenosis in the proximal LAD, which was thought to be responsible for the ischemic symptom. In 2006, he again underwent CAG for chest pain, and this showed an intact stent in the proximal LAD and no change in the MB of the mid-LAD, which showed moderate systolic stenosis (50% luminal narrowing during systole) and total compressed length was 22.5 mm long (Fig. 1A). On an outpatient basis, his blood pressure (BP) ranged from 120-130/80-90 mmHg and his pulse was 60-70 beats per minute. He was being treated with 100 mg of aspirin, 5 mg of bisoprolol and 8 mg of perindopril, which he tolerated well without chest pain.
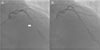
At his visit in 2014, the patient experienced a squeezing chest pain accompanied by uncontrolled hypertension. He had stopped taking medication three months before admission. On admission, his BP was 160/100 mmHg and his pulse rate was 94 beats per minute. Cardiac markers were within normal limits (creatinine kinase muscle-brain fraction 1.40 ng/mL, troponin-I <0.01 ng/mL). Twelve leads electrocardiography showed normal sinus rhythm with a right bundle branch block, which had not changed compared to the previous study. On echocardiography, the left ventricular ejection fraction was in the normal range and there are no regional wall motion abnormalities. Left ventricular wall thickness also had not changed compared to the previous result. Follow-up CAG revealed no in-stent restenosis of the proximal LAD stent and no new atherosclerotic lesions that might cause ischemic symptoms. However, the MB at the mid-LAD had aggravated to severe stenosis (>90% luminal narrowing during systole) and total compressed length was extended to 23.9 mm (Fig. 2A). The coronary blood flow was disturbed in systole and recovered in diastole. These findings suggested aggravation of the MB and his ischemic symptom originated from this aggravation. After his BP was controlled at <130/80 mmHg and heart rate was reduced to 70 beats per minute with regular administration of bisoprolol and perindopril, he did not experience chest pain anymore. He was discharged from the hospital with medications and no symptoms have been observed during follow-up at the outpatient clinic.
MB can be caused by systolic compression of the coronary artery encircled with the myocardium.13 Although the prognosis of MB is generally good, a considerable number of patients with MB were reported to experience acute myocardial infarction, sudden death, and coronary angina over time.256 It has been suggested that the clinical spectrum of MB can be broader. Delayed diastolic relaxation and peak coronary blood flow can be observed in patients with MB, which can decrease coronary vasodilator reserve.7 This is the most important mechanism causing myocardial ischemia. Faruqui et al. suggested that three factors contribute to the development of MB in patients with myocardial bridging, which include length of the tunneled coronary segment, degree of systolic compression, and heart rate.4 Myocardial hypertrophy is related to the depth and length of the tunneled segment, and the degree of systolic compression can be influenced by BP or sympathetic drive. Tachycardia from sympathetic drive during stress or exercise likely facilitates ischemia, because it leads to an increase of the systolic-diastolic time ratio at the expense of diastolic flow.3
However, there are few reports of the change in the degree of MB in CAG. Yano et al. reported that recovery of MB occurred after improving left ventricular function in patients with acute coronary syndrome.8 While, Masuda et al. reported the aggravation of MB on follow-up CAG.9 These reports suggest that the severity of MB can be changed in CAG depending on left ventricular hemodynamics and myocardial contractility and this change is related to myocardial ischemia. In our case, the aggravation of the narrowing and the length of tunneled artery was proved by CAG, which resulted in an ischemic cardiac symptom. With poor compliance, the patient had sustained high BP and increased pulse rate until admission. Although there was no significant change of left ventricular wall thickness on echocardiography, uncontrolled hypertension could induce localized ventricular hypertrophy through the increase of afterload and myocardial contractility. This mechanism might cause more severe systolic compression and longer bridged segment in MB. Increased length and systolic compression of a tunneled artery can be explained by progression of localized hypertrophy at the bridged segment.910 Increased pulse rate can also facilitate myocardial ischemia. After his BP and pulse rate stabilized, he had no ischemic symptoms. This suggests that control of myocardial contractility and pulse rate is an essential treatment for severe MB to prevent the development of myocardial ischemia. In this context, beta blockers and angiotensin converting enzyme inhibitors can be important medications for preventing the aggravation of MB.
Limitation of this case report was that there is no direct evidence of myocardial ischemia proven by stress test such as stress echocardiography and single-photon emission imaging tomography before the CAG. As a result, myocardial ischemia in LAD territory has to be inferred by clinical situation derived from the patient's symptom and angiographic result.
In conclusion, MB can be aggravated in terms of stenosis and length, and it can cause myocardial ischemia. This is the first case report to prove the aggravation of MB by CAG in Korea. Although the exact mechanism was not well established, increased myocardial contractility, tachycardia and local ventricular hypertrophy were believed to have important roles in the aggravation. Consequently, it is important to control myocardial contractility and heart rate with regular medication, such as beta blockers and angiotensin converting enzyme inhibitors to prevent the aggravation of MB and myocardial ischemia. However, there are insufficient data for treatment of the worsening of MB, and further investigations are needed to uncover the detailed mechanism and proper prevention.
Figures and Tables
References
2. Mazzù A, Di Tano G, Cogode R, Lo Presti G. Myocardial bridging involving more than one site of the left anterior descending coronary artery: an uncommon cause of acute ischemic syndrome. Cathet Cardiovasc Diagn. 1995; 34:329–332.

3. Möhlenkamp S, Hort W, Ge J, Erbel R. Update on myocardial bridging. Circulation. 2002; 106:2616–2622.

4. Faruqui AM, Maloy WC, Felner JM, Schlant RC, Logan WD, Symbas P. Symptomatic myocardial bridging of coronary artery. Am J Cardiol. 1978; 41:1305–1310.

5. Cutler D, Wallace JM. Myocardial bridging in a young patient with sudden death. Clin Cardiol. 1997; 20:581–583.

6. Arjomand H, AlSalman J, Azain J, Amin D. Myocardial bridging of left circumflex coronary artery associated with acute myocardial infarction. J Invasive Cardiol. 2000; 12:431–434.
7. Erbel R, Rupprecht HJ, Ge J, Gerber T, Görge G, Meyer J. Coronary artery shape and flow changes induced by myocardial bridging. Echocardiography. 1993; 1:71–77.

8. Yano K, Yoshino H, Taniuchi M, Kachi E, Shimizu H, Watanuki A, et al. Myocardial bridging of the left anterior descending coronary artery in acute inferior wall myocardial infarction. Clin Cardiol. 2001; 24:202–208.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download