Abstract
The kinesin superfamily is a class of motor proteins moving along microtubule filaments and playing essential roles in mitosis of eukaryotic cells. In the cancer biology, mitotic activity is an essential factor for development and metastasis of various cancers. Therefore, the inhibition of kinesin activity is suggested as an alternative cancer therapy. Accumulated clinical evidences have proved the potency of kinesin inhibitors in cancer treatments. In this review, we provided an overview of kinesins that play a critical role in the pathophysiology of various cancers and described the beneficial vs. side effects of their inhibitors that have been tested in both basic science and clinical studies.
Cancer is a gene-associated disease with abnormal and unregulated cell proliferation. Normal cell growth needs a balance between the self-activities of those genes that control cell proliferation. It also depends on the activities of genes that signal when damaged cells should undergo cell death. In case of healthy cells, few thousand of genes activity control the process of cell division. The cell division means to divide into two or more daughter cells, that daughter cell retains with a full set of chromosomes. Agents targeting cell division could induce abnormal mitosis in tumor cells, may lead to mitotic arrest and cell death. The Kinesin superfamily is a protein belongs to a class of motor proteins, that kinesins move along microtubule filaments, and are powered by the hydrolysis of adenosin triphosphate (ATP). The Kinesin plays an essential roles in mitosis of eukaryotic cells, such as mitotic spindle function, targets in the anti-mitotic cancer therapeutics.
Some that have already been studied in phase I/II clinical trials have shown anti-proliferative effects without causing significant neuropathy. Because kinesin spindle protein (KSP) inhibitors do not target microtubules, they are more specific and associated with lesser side effects then anti-microtubule drugs. In this review, we will provide an overview of kinesins that play a critical role in the pathophysiology of various cancers and describe the beneficial vs. side effects of their inhibitors that have been tested in both basic science and clinical studies.
Kinesin was discovered in 1985 from nervous tissue.1 Kinesins generate directed mechanical force by hydrolyzing ATP and move along microtubule filaments from the minus end (oriented toward the nucleus) to the plus end (oriented toward the cell periphery).2 Kinesins plays a massive role in the separation of centrosomes, the assembly of bipolar spindles, and the faithful segregation of chromosomes into daughter cells during prophase, prometaphase, metaphase, anaphase, and telophase.3 Kinesins also serve an important role in microtubule polymer dynamics, and signal transduction.4 Mitotic kinesins are cytoskeletal motor proteins that perform special and essential roles in cell proliferation. These proteins transport cellular components including genetic materials along microtubule. So KSP inhibitors specifically disrupt cell division, leaving other cytoskeletal processes unaffected, and therefore represent a potentially safer more effective approach to the treatment of cancer.
Around 650 kinesin superfamily discovered in all eukaryocytic organisms. The Kinesin motor proteins often participate in cell- and tissue specific functions, such as mitosis or meiosis.5 Over than 40 kinesin proteins, named kinesin-1 through kinesin-14.6,7 kinesin-1,3,4,12 and kinesin-14 are transport of organelles, whereas kinesin-1, kinesin-4,5,6,7,8,10,12 and kinesin-13 participated in cell mitosis, particularly in spindle formation, cytokinesis, chromosomal separation and nuclear movement.8 In clinic, overexpression of KSP had been used as a diagnostic marker of several cancers including bladder, stomach, breast, lung and colorectal cancers and survival rate of patients.9,10,11,12,13,14,15 Because cancer is a gene-associated disease with uncontrolled cell growth, targeting kinesins may develop a novel therapeutic strategy cancers. To date several KSP inhibitors have been successfully studied in clinical trials.
Most of the kinesins are plays an essential role for cancer diagnosis and prognostic marker, also some of kinesins have been implicated in a variety of disease. Kinesin superfamily (KIF) member Kinesin 4, such as KIF 4A gene is important prognostic marker for lung cancer. Taniwaki et al12 identified that KIF4A gene expression around 5 fold in small cell lung cancer (SCLC), however around 40% of non-small cell lung cancer (NSCLC). Also high expression of KIF4 gene detected in cervical cancer16 and essential role in the some cancers, such as glioma, melanoma, breast and bladder cancer.17,18 By contrast, KIF4 downregulation observed in gastric carcinoma.19 Overexpression of KIF14 promotes the development of breast and lung cancer. However, in case of retinoblastoma cancer, the KIF14 expressed more than two times20,21 and has been studied as a prognostic marker or for disease-free survival in breast and lung cancer.20 Kinesin-13 family member, mitotic centromere-associated kinesin (MCAK) is participates in mitosis.22 MCAK overexpressed in many kinds of cancers, such as breast, colorectal cancer and glioma tissues,23,24 therefore it can act as a prognostic marker in colon cancer. Nishidate et al.,25 reported that high-expression of MCAK leads to breast cancer development, also can be suppressed by p53.26 Ishikawa et al., mentioned MCAK as a marker for poor prognosis of lymph node metastasis in colorectal cancer.27 KIF20B (MPHOSPH1) is high expressed in bladder and colon cancer and cytokinesis defects depending by downregulation of KIF20B.18,28 Wang et al., reported that KIF2A strongly expressed in metastasis of squamous cell carcinoma of the tongue (SCCOT) and lymph node metastasis.18,29 KIF18 kinesins have been shown important role in chromosome congression. Mitotic kinesin KIF18 was found overexpressed in colorectal and breast cancer. It is associated with tumour grade metastasis.30,31 Upregulation of mitotic kinesin-like motor protein (MKLP)1 was shown NSCLC32 and hepatocellular carcinoma.33 Moreover MKLP2 high expressed in pancreatic ductal adenocarcinoma (PDAC), and inhibited the growth of gastric cancer cells.34 High-expression of KIFC3 in docetaxel resistance in breast cancer cells.35
Corson et al., investigated that KIF14 overexpression is thus tumor-specific such as breast cancer. Also it was predicted decreased disease-free survival20. Moreover, mitotic kinesin KIF14 showed genomic gain and high expressed in many cancers including ovarian cancer. Theriault B et al., investigated that expression of the KIF14 is predictive of poor prognosis in breast and lung cancer.36 The mitotic kinesin KIF1B mutations were detected in pheochromocytomas and neuroblastomas. Also KIF1B beta germline variant related neural crest and nonneural origin.37 Liu et al., reported the overexpression Eg5 in human pancreatic cancer, and high expression of Eg5 was clinicopathological parameters of pancreatic cancer.38,39
Kanehira et al., reported that MPHOSPH1 genes were significantly overexpressed in the majority of bladder cancers.28 In fact about KIF23 downregulated in glioma cells, so Takahash et al., suggested that KIF23 might be a novel therapeutic agent of malignant glioma.40 KIF20A kinesins downregulation inhibits the growth of gastric cancer, also can play an essential role in anti-cancer mechanism of genistein.41
CENP-E plays an important role in the function of spindle checkpoint. Lui et al., discovered that the down expression of CENP-E in human hepatocellular carcinoma.42 However another researchers reported that high expression of CENP-E associated with poor prognosis in breast cancer.43
Kif18A is a microtubule depolymerase and plays an essential role of chromosome congregation. Overexpression of Kif18A was found in colorectal cancer and metastasis.31 Bie et al., established KIF2C was high expressed in glioma and as a potential independent prognostic parameter for patient with glioma.23 In addition Ishikawa et al., reported overexpression of MCAK has been associated with aggressive forms of carcinoma and a key prognosis of colorectal cancer.27
Cancer can occur when the balance between mitosis and apoptosis is imbalanced or disrupt. Previously it has been well demonstrated that KSP inhibitors lead to mitotic arrest and cell death.58,59 Kinesin-5 proteins a sensitive to battery of small molecule inhibitors that allosterically block Eg5 acitivity.58,60,61 Since its discovery, over 100 different chemical classes of allosteric inhibitors against HsEg5 have been identified in the public scientific literature. These include carbolines, quinazolines, thiazolopyrimidines, thiadiazoles, dihydropyrazoles, isoquinolines, imidazoles, and benzimidazoles.62,63,64 A few years ago, Mayer et al., discovered monastrol shown to inhibit the kinesin Eg5, arrested cells are characterized by monopolar spindle. This phenotype is induced through specific disruption of mitotic molecular motor kinesin Eg5 with IC50 at 14 µM. No effect on other motor proteins and tubulin. This discovery regarded as a paradigm shift in anticancer drug development.65
Ispinesib (SB 715992) was the first KSP inhibitor that entered clinical trials. Ispinesib shown to inhibit cancer cell proliferation of human and murine cell lines with IC50 values of 1.2-9.5 nM. Also have been shown cytotoxic activity in tumor cell lines, including Colo201, Colo205, HT-29, M5076, Madison-109, and MX-1, with IC50 of 1.2 nM to 9.5 nM.66 20 nM ispinesib caused mitotic arrest in SKOV3 ovarian tumor cells, which displayed unseparated centrosomes and monopolar mitotic spindles. It also showed favorable clinical activity with a significantly lower toxicity profile in SKOV3 human tumor xenograft models, compared to tubulin targeted drugs. In addition, it exhibited anti-proliferative activity in tumor cell lines and has been shown to be effective in several murine tumor models.66 To note, the clinical trial with KSP inhibitor ispinesib was rather disapponting. Another facts, Ispinesib and MK-0731 have shown antiproliferative activity in tumor cell lines and several murine tumor models.67,68
A new generation of SB743921 exhibited five-fold increased in potency against Eg5 over ispinesib with > 40,000-fold selectivity for KSP over other kinesin.62 It exhibited strong cytotoxic activity in vitro and in vivo. SB 743921 confirmed a functional mitotic spindle causing G2/M cell cycle arrest and apoptosis. SB743921 have been shown cytotoxic activity against xenograft models and cancer cell lines, such as Colo205 (complete regressions), MX-1, SKOV3, MV522 and P388, with IC50 of 0.2 nM to 14.4 nM.69 In 2009, Woessner et al., reported a thiadiazole derivative ARRY-5220 demonstrated potent anti-proliferative activity in epithelial ovarian cancer cells.70
KSP inhibition by ARRY-520 results in various beneficial effects against cancer development. The rectification of chromosome segregation may be activated via the spindle assembly checkpoint. It may also result in the interruption of the cell cycle during mitotic phase, which may then cause cell death to actively-dividing cells. Moreover, it does not cause peripheral neuropathy, a disease typically associated with tubulin-targeting agents, since KSP does not participate in events post mitosis. ARRY-520 showed beneficial inhibitory activity against several cell lines (HT-29, HCT-116, A2780, K562 and HCT-15).71 Previous studies demonstrated that ARRY-520 blocked cell cycle in mitosis and caused apoptosis in AML cell lines.62,72 SCH 2047069 showed antitumor activity in several solid tumor xenograft models, including human ovarian carcinoma A2780, human colon carcinoma Colo-205, human glioblastoma U373, and colon carcinoma HCT-116. SCH 2047069 was also significant active in EOL-1 leukemia and DoHH2 lymphoma models and significantly extended survival of these animals.73
HR22C16 is required for cell division (IC50=800±10 nm).74 Marcus et al, tested to combination of HR22C16A with Paclitaxel.75 Just few years ago, another researchers were also checked anti-proliferative effect in human lung cancer H1299 cells and human lung fibroblast WI38 cells.76 They demonstrated that HR22C16A showed efficacy effect on cell death and mitotic arrest in cancer cells. A new compound CPUYL064 showed great inhibitory effect against Eg5. Yang et al., reported CPUYL064 was found G2/M phase arrest and anti-tumor activity against human hepatocellular carcinoma cell line with IC50 of 100 nM.77
K858 is a thiadiazole derivative with KSP IC50 value of 1.3 µM. K858 displayed potent inhibitory activity both in vitro against HCT116 cell line and in vivo in many xenograft models of cancer.78 The oral KSP inhibitor SCH 2047069 has been shown the ability to cross the blood-brain barrier.73 SCH 2047069 exhibited anti-tumor activity against leukemia, lymphoma, ovarian and colorectal cancers both in vitro (57 tumor cell lines) and in vivo. It could also enhance the antitumor activity in A2780 xenograft models combination with gemcitabine, vincristine and paclitaxel.79
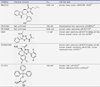
Dihydropyrrole derivative MK-0731 is a synthetic small molecule and a potent and selective KSP (IC50=2.2 nM),80 Furthermore, MK-0731 which displayed high efficacy anti-proliferative activity in mouse xenograft model.68 The discovery of a benzimidazole derivative CPUYJ039 is a novel and potent KSP inhibitor (IC50=0.04 µM) was reported in 2011. CPUYJ039 blocked cell cycle in G2/M phase and leading to cell death with the monastrol spindle phenotype in HCT116 cell line.80 ARQ 621 demonstrated anti-tumor activity against a wide range of human cancer cell lines in vitro, including colon, lung, endometrial, bladder, and hematologic cancer cell lines and in a number of xenografts grown in athymic mice, including pancreatic, breast, prostate, and ovarian carcinomas. Compared to ispinesib, a previously characterized Eg5 inhibitor, ARQ 621 demonstrated comparable potency and proved to be a non-DNA damaging agent.81 Table 2 shows that activities of KSP inhibitors in vitro studies.
Inhibition of KSP leads to apoptosis of several cancer cell lines as well as in vitro anti-tumor activity in human xenograft models.78,84,85 Leizerman et al., have demosntrated that monastrol causes mitotic arrest and caspase activated cell death (activation of caspase-8, caspase-3 activation, cleavage of PARP) and mitochondria dysfunction in human gastric AGS and colon HT29 cell lines.86 Vijapurkar et al., further elucidated the cellular responses following monastrol-induced mitotic arrest.87 They suggest that the cellular responses induced by monastrol that are correlated with overexpression of BclXL, the antiapoptotic Bcl-2 family protein. Overexpression of BclXL provides a protective mechanism, and its depletion rescues the apoptotic response to monastrol.88 Liu et al., observed that monastrol inhibitor arrested mitosis, induced cell death, and overexpressed Hsp70 (heat shock protein) in human multiple myeloma cells.89 ARRY-520 treated Type II EOC cells exhibited a significantly increased caspase family such as caspases-8, 9, 3 and decreased of XIAP.70 Caspase-2 activation leads to involvement of the mitochondria. In addition, Tunquist et al., showed that ARRY-520 treated in human multiple myeloma NCI H929 cells observed overexpressed cleaved-PARP during mitotic arrest, and decreased of the antiapoptotic protein myeloid cell leukemia 1 (Mcl-1).90 Kinesin-5 in HL60 cells showed intrinsic apoptosis during mitotic arrest with mitochondrial dysfunction such as loss of mitochondrial membrane potential and upstream event of MOMP.91 Furthermore, Shimizu et al., reported that KSP inhibitor CF3-STLC induced mitotic arrest with the monoastral spindles, and cell death with cleavage of PARP-1, caspase-3, and 4E-BP1.82 Rather Ogo et al., reported same result in HeLa cell.83 Recently, Basso et al., found that HT-29 cells with oral KSP inhibitor SCH 2047069 led to mitotic arrest and cell death with PARP cleavage.73 CPUYJ039 inhibited HCT116 cells proliferation, and G2/M cell-cycle arrest with characteristic monoastral spindles. Also showed that cell death with an increase of the Bax/Bcl-2 ratio in HCT116 cells80 However Tao et al., demonstrated that apoptosis after prolonged mitotic arrest with activated pro-apoptotic protein Bax. The KSP-IA,92 a dihydropyrrole small molecule arrests cells in mitosis and induces apoptosis by caspase-dependent death. Moreover, KSP-IA was able to induce apoptotic cell death in a p53-independent manner, suggesting that KSP inhibitors could be proved active in p53-deficient tumors.
Orth et al., investigated that prolonged mitotic arrest activated the intrinsic apoptotic pathway. That activates p53 induction after slippage with mitochondrial dysfunction and caspase activated DNAse, causing limited DNA damage in human cancer cells.93 Hwang et al., showed that KSP inhibitor HR22C16 sensitized in H1299 cells to TRAIL-induced cell death by down-regulating XIAP, survivin and Bcl-2 apoptotic proteins and the activity of NF-κB.76 Recently, Yin et al., demonstrated that SB743921 treatment suppresses the ERK and Akt activity in CML cells.94
In fact, a lot of new KSP inhibitors have been identified in recent years. Some of the inhibitors have already been studied in phase I or Phase II clinical trials and proved to have shown anti-proliferative effects in several types of cancers. KSP inhibitors have been successful when used as a monotherapy. The Ispinesib (SB715992) was the first KSP inhibitor to enter clinical trials.66 At present, it represents the most advanced and best studied KSP inhibitor. Phase I studies of ispinesib in patients with solid tumors have been completed. Generally, ispinesib was well-tolerated with no indications of neurotoxicity. The most common adverse effects were neutropenia, fatigue, anemia, leukopenia, thrombocytopenia, diarrhea, nausea, and vomiting.95,96,97 The most promising results have been observed in patients with advanced or metastatic breast cancer.98 Recently, outcomes from the phase II trial of ispinesib99,100,101,102,103 in 15 patients with metastatic hepatocellular carcinoma were published.104 Similar results were obtained from the phase II study in patients with melanoma.105 Other preliminary reports of ispinesib in metastatic squamous cell carcinoma of the head and neck,99 colorectal cancer, ovarian cancer, and renal cell carcinoma have not indicated significant response rates.18 Despite some promising results the outcomes from the first clinical trials of ispinesib are rather disappointing. It is known that ispinesib resistance, observed in some clinical trials, may be due to multidrug resistance.106,107 Taken together, clinical trials evaluating the efficacy and safety of KSP inhibitors, both as single and in combination with other agents, are currently ongoing. Second-generation SB743921 is now in the clinic use. SB743921, a derivative of ispinesib, is 5-fold more potent against KSP ATPase activity and it is another promising KSP inhibitor from Cytokinetics. SB743921 exhibited five-fold increase in potency against KSP over ispinesib with > 40,000-fold69 and is entered in a phase I/II clinical trial in non-Hodgkin's lymphoma.62 Now its undergoing Phase I clinical study. SB743921 showed response in some patients and the major dose limiting toxicity was marrow suppression, in particular neutropenia.62 MK-0731 is a dihydropyrrole derivative developed by Merck and ARRY-520 is a thiadiazole derivative from Array Pharmaceuticals. MK-0731 (Merck) progressed in clinical development has shown anti-proliferative activity in several tumor cell lines and significant efficacy in some murine tumor models. MK-0731 was tolerated when administrated in 17 mg/m2 dose.62 In addition, ARRY-520 (Array Biofarma) is currently in a phase I trial and phase II in advanced cancer patients and has shown remarkable efficacy in preclinical models of human solid tumors and human leukemia showed only limited response.108
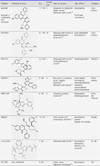
The AZD4877,109,110 MK-0731111 and EMD544085 as novel KSP single agent in melanoma105 and clear-cell renal cell cancer.39,112 However the final clinical results have not been reported. Therapy with ARQ621 appears well tolerated, with no dose-limiting toxicity observed at doses and frequencies much higher than those achieved with the leading Eg5 inhibitors of comparable potencies. Wakui et al., studied effect of LY2523355 in Japanese patients with advanced solid tumors. Who had grade 4 neutropenia or grade 3 febrile neutropenia, they used G-CSF. Also observed that no objective tumor responses.113 The most frequent side effects were neutropenia, leukopenia, diarrhea, rash and mucositis.114 4SC-205 is now being investigated in a clinical phase 1, in patients with solid tumors or malignant lymphomas.115 The compound exhibits dose-proportional pharmacokinetics with t 1/2 ~10. Dose limiting toxicities include increased neutropenia.116 Table 3 shown KSP inhibitors in clinical development and some side effects.
Drug resistance is common cause of treatment failure for cancer. Resistance to chemotherapeutic drugs is a big problem of treatment of patients with cancer that encumbers the efficacy of cytostatic drugs.18 Which may be caused through the expression of efflux pumps such as P-gp121,122 and several KIF proteins, such as KIFC3 and MCAK, might be lead to drug resistance.54,57
The way to target KIF combined with chemotherapy can provide a classic manner to treat cancer, when serious chemotherapeutic durg resistance exist then cancer cells' anti-apoptotic proteins can also generate drug resistance.18 Preclinical studies mentioned that Ispinesib is predicted to be a substrate for drug resistance. A KSP inhibitor developed by Merck, MK-0731, has been modified and avoided to be the substrate for P-glycoprotein mediated efflux, giving it more potential for entering clinical trials. Binding pocket mutation is another origin of drug resistance in cancers. An alteration of the L5 pocket of KSP could offset the action of APT non-competitive inhibitors binding to it. Prior to patient use, this mutation could be measured by structural biomarkers predictive of drug potency.123
Neutropenia is the most common side effects of KSP inhibitors and along other side effects. Because the doubling time of granulocyte precursors is very short [17 hours for myeloblasts, 63 hours for promyelocytes, and 55 hours for myelocytes]. Thus, reversible neutropenia would be expected of an agent targeting a mitotic kinase or KSP, because at any one time ~25% of bone marrow neutrophils are undergoing mitosis.124 Other common side effects are anemia, fatigue, nausea/vomiting and leukopenia, overexpression of aspartate or alanine aminotransferase, hyperbilirubinemia and hyponatremia. Clinical studies for AZD4877 and MK0731 have been carried out because of leukopenia, elevation of aspartate and alanine aminotransferase, hyperbilirubinemia and hyponatremia.108,109,119,125 Some cases, no neurotoxicity observed in trials.95,110,119 Because some KSP is expressed at low levels in the adult nervous system in rodent and KSP inhibitors do not target microtubules.126
Going back to 1985 when the first kinesin was discovered with 14 kinesin family types, each was identified that contains various proteins. Alteration of these protein expression and functions leads to human disease, including tumorgenesis and progression. KSP inhibitors are attractive and promising anti-proliferative agents for cancer chemotherapy, such as some solid tumors and hematologic malignancies. Compared with other antimitotics, KSP inhibitors have shown potent anti-proliferative effects without causing significant neuropathy. The challenge for clinical development of KSP inhibitors observed drug resistance and side effects. However novel KSP inhibitors and combination therapy is still attractive field. Another unanswered question is how do KSP inhibitors induce mitochondria mediated cell death? Although it is suggested that KSP inhibition enhances caspase mediated apoptotic cell death but the underlying mechanism is not yet clear. These concerns are needed to be answered in the future studies. Besides blocking tumor cell proliferation, kinesin inhibitors showed potential to block angiogenesis127 which further suggest novel mechanism of kinesin inhibitors in pathophysiological modulation of vascular forming and functions.
Figures and Tables
Table 1
Kinesin subfamily protein assocaited tumorogenesis and function

This table is adapted from the Review article of Xinran Liu et al.18
ACKNOWLEDGEMENT
This study was supported by a grant from the National R&D Program for Cancer Control, Ministry for Health, Welfare and Family affairs, Republic of Korea (0920040) and Priority Research Centers Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010-0020224).
References
1. Vale RD, Reese TS, Sheetz MP. Identification of a novel force-generating protein, kinesin, involved in microtubule-based motility. Cell. 1985; 42:39–50.

2. Rowinsky EK, Chaudhry V, Cornblath DR, Donehower RC. Neurotoxicity of Taxol. J Natl Cancer Inst Monogr. 1993; 107–115.
3. Howard J. Mechanics of motor proteins and the cytoskeleton. Sunderland (MA): Sinauer Associates, Inc.;2001.
4. Goldstein LS, Philp AV. The road less traveled: emerging principles of kinesin motor utilization. Annu Rev Cell Dev Biol. 1999; 15:141–183.

5. Wordeman L. How kinesin motor proteins drive mitotic spindle function: lessons from molecular assays. Semin Cell Dev Biol. 2010; 21:260–268.

6. Miki H, Setou M, Kaneshiro K, Hirokawa N. All kinesin superfamily protein, KIF, genes in mouse and human. Proc Natl Acad Sci U S A. 2001; 98:7004–7011.

7. Lawrence CJ, Dawe RK, Christie KR, Cleveland DW, Dawson SC, Endow SA, et al. A standardized kinesin nomenclature. J Cell Biol. 2004; 167:19–22.

8. Yu Y, Feng YM. The role of kinesin family proteins in tumorigenesis and progression: potential biomarkers and molecular targets for cancer therapy. Cancer. 2010; 116:5150–5160.

9. Li L, Li XQ, Pan XH, Feng YM. Prognostic prediction by detection of KIF1B mRNA level in breast cancer and its clinical significance. Chin J Breast Dis. 2009; 173–180.
10. Corson TW, Zhu CQ, Lau SK, Shepherd FA, Tsao MS, Gallie BL. KIF14 messenger RNA expression is independently prognostic for outcome in lung cancer. Clin Cancer Res. 2007; 13:3229–3234.

11. Feng YM, Wan YF, Li XQ, Cao XC, Li X. Expression and clinical significance of KNSL4 in breast cancer. Ai Zheng. 2006; 25:744–748.
12. Taniwaki M, Takano A, Ishikawa N, Yasui W, Inai K, Nishimura H, et al. Activation of KIF4A as a prognostic biomarker and therapeutic target for lung cancer. Clin Cancer Res. 2007; 13:6624–6631.

13. Schlisio S, Kenchappa RS, Vredeveld LC, George RE, Stewart R, Greulich H, et al. The kinesin KIF1Bbeta acts downstream from EglN3 to induce apoptosis and is a potential 1p36 tumor suppressor. Genes Dev. 2008; 22:884–893.

14. Castillo A, Morse HC 3rd, Godfrey VL, Naeem R, Justice MJ. Overexpression of Eg5 causes genomic instability and tumor formation in mice. Cancer Res. 2007; 67:10138–10147.

15. Cardoso CM, Groth-Pedersen L, Høyer-Hansen M, Kirkegaard T, Corcelle E, Andersen JS, et al. Depletion of kinesin 5B affects lysosomal distribution and stability and induces peri-nuclear accumulation of autophagosomes in cancer cells. PLoS One. 2009; 4:e4424.

16. Narayan G, Bourdon V, Chaganti S, Arias-Pulido H, Nandula SV, Rao PH, et al. Gene dosage alterations revealed by cDNA microarray analysis in cervical cancer: identification of candidate amplified and overexpressed genes. Genes Chromosomes Cancer. 2007; 46:373–384.

17. Rouam S, Moreau T, Broët P. Identifying common prognostic factors in genomic cancer studies: a novel index for censored outcomes. BMC Bioinformatics. 2010; 11:150.

18. Liu X, Gong H, Huang K. Oncogenic role of kinesin proteins and targeting kinesin therapy. Cancer Sci. 2013; 104:651–656.

19. Gao J, Sai N, Wang C, Sheng X, Shao Q, Zhou C, et al. Overexpression of chromokinesin KIF4 inhibits proliferation of human gastric carcinoma cells both in vitro and in vivo. Tumour Biol. 2011; 32:53–61.

20. Corson TW, Gallie BL. KIF14 mRNA expression is a predictor of grade and outcome in breast cancer. Int J Cancer. 2006; 119:1088–1094.

21. Madhavan J, Coral K, Mallikarjuna K, Corson TW, Amit N, Khetan V, et al. High expression of KIF14 in retinoblastoma: association with older age at diagnosis. Invest Ophthalmol Vis Sci. 2007; 48:4901–4906.

22. Maney T, Hunter AW, Wagenbach M, Wordeman L. Mitotic centromere-associated kinesin is important for anaphase chromosome segregation. J Cell Biol. 1998; 142:787–801.

23. Bie L, Zhao G, Wang YP, Zhang B. Kinesin family member 2C (KIF2C/MCAK) is a novel marker for prognosis in human gliomas. Clin Neurol Neurosurg. 2012; 114:356–360.

24. Gnjatic S, Cao Y, Reichelt U, Yekebas EF, Nölker C, Marx AH, et al. NY-CO-58/KIF2C is overexpressed in a variety of solid tumors and induces frequent T cell responses in patients with colorectal cancer. Int J Cancer. 2010; 127:381–393.

25. Nishidate T, Katagiri T, Lin ML, Mano Y, Miki Y, Kasumi F, et al. Genome-wide gene-expression profiles of breast-cancer cells purified with laser microbeam microdissection: identification of genes associated with progression and metastasis. Int J Oncol. 2004; 25:797–819.
26. Shimo A, Tanikawa C, Nishidate T, Lin ML, Matsuda K, Park JH, et al. Involvement of kinesin family member 2C/mitotic centromere-associated kinesin overexpression in mammary carcinogenesis. Cancer Sci. 2008; 99:62–70.

27. Ishikawa K, Kamohara Y, Tanaka F, Haraguchi N, Mimori K, Inoue H, et al. Mitotic centromere-associated kinesin is a novel marker for prognosis and lymph node metastasis in colorectal cancer. Br J Cancer. 2008; 98:1824–1829.

28. Kanehira M, Katagiri T, Shimo A, Takata R, Shuin T, Miki T, et al. Oncogenic role of MPHOSPH1, a cancer-testis antigen specific to human bladder cancer. Cancer Res. 2007; 67:3276–3285.

29. Wang CQ, Qu X, Zhang XY, Zhou CJ, Liu GX, Dong ZQ, et al. Overexpression of Kif2a promotes the progression and metastasis of squamous cell carcinoma of the oral tongue. Oral Oncol. 2010; 46:65–69.

30. Zhang C, Zhu C, Chen H, Li L, Guo L, Jiang W, et al. Kif18A is involved in human breast carcinogenesis. Carcinogenesis. 2010; 31:1676–1684.

31. Nagahara M, Nishida N, Iwatsuki M, Ishimaru S, Mimori K, Tanaka F, et al. Kinesin 18A expression: clinical relevance to colorectal cancer progression. Int J Cancer. 2011; 129:2543–2552.

32. Välk K, Vooder T, Kolde R, Reintam MA, Petzold C, Vilo J, et al. Gene expression profiles of non-small cell lung cancer: survival prediction and new biomarkers. Oncology. 2010; 79:283–292.

33. Wang SM, Ooi LL, Hui KM. Upregulation of Rac GTPase-activating protein 1 is significantly associated with the early recurrence of human hepatocellular carcinoma. Clin Cancer Res. 2011; 17:6040–6051.

34. Taniuchi K, Nakagawa H, Nakamura T, Eguchi H, Ohigashi H, Ishikawa O, et al. Down-regulation of RAB6KIFL/KIF20A, a kinesin involved with membrane trafficking of discs large homologue 5, can attenuate growth of pancreatic cancer cell. Cancer Res. 2005; 65:105–112.
35. De S, Cipriano R, Jackson MW, Stark GR. Overexpression of kinesins mediates docetaxel resistance in breast cancer cells. Cancer Res. 2009; 69:8035–8042.

36. Thériault BL, Pajovic S, Bernardini MQ, Shaw PA, Gallie BL. Kinesin family member 14: an independent prognostic marker and potential therapeutic target for ovarian cancer. Int J Cancer. 2012; 130:1844–1854.

37. Yeh IT, Lenci RE, Qin Y, Buddavarapu K, Ligon AH, Leteurtre E, et al. A germline mutation of the KIF1B beta gene on 1p36 in a family with neural and nonneural tumors. Hum Genet. 2008; 124:279–285.

38. Liu M, Wang X, Yang Y, Li D, Ren H, Zhu Q, et al. Ectopic expression of the microtubule-dependent motor protein Eg5 promotes pancreatic tumourigenesis. J Pathol. 2010; 221:221–228.

40. Takahashi S, Fusaki N, Ohta S, Iwahori Y, Iizuka Y, Inagawa K, et al. Downregulation of KIF23 suppresses glioma proliferation. J Neurooncol. 2012; 106:519–529.

41. Yan GR, Zou FY, Dang BL, Zhang Y, Yu G, Liu X, et al. Genistein-induced mitotic arrest of gastric cancer cells by downregulating KIF20A, a proteomics study. Proteomics. 2012; 12:2391–2399.

42. Liu Z, Ling K, Wu X, Cao J, Liu B, Li S, et al. Reduced expression of cenp-e in human hepatocellular carcinoma. J Exp Clin Cancer Res. 2009; 28:156.

43. Agarwal R, Gonzalez-Angulo AM, Myhre S, Carey M, Lee JS, Overgaard J, et al. Integrative analysis of cyclin protein levels identifies cyclin b1 as a classifier and predictor of outcomes in breast cancer. Clin Cancer Res. 2009; 15:3654–3662.

44. Dyrskjøt L, Kruhøffer M, Thykjaer T, Marcussen N, Jensen JL, Møller K, et al. Gene expression in the urinary bladder: a common carcinoma in situ gene expression signature exists disregarding histopathological classification. Cancer Res. 2004; 64:4040–4048.
45. Richardson AL, Wang ZC, De Nicolo A, Lu X, Brown M, Miron A, et al. X chromosomal abnormalities in basallike human breast cancer. Cancer Cell. 2006; 9:121–132.

46. Jimbo T, Kawasaki Y, Koyama R, Sato R, Takada S, Haraguchi K, et al. Identification of a link between the tumour suppressor APC and the kinesin superfamily. Nat Cell Biol. 2002; 4:323–327.

47. Lukong KE, Richard S. Breast tumor kinase BRK requires kinesin-2 subunit KAP3A in modulation of cell migration. Cell Signal. 2008; 20:432–442.

48. Corson T, Zhu C, Lau S, Shepherd F, Tsao M, Gallie B. KIF14 messenger RNA expression is independently prognostic for outcome in lung cancer. Clin Cancer Res [serial on the Internet]. 2007; 13:Available from: http://www.ncbi.nlm.nih.gov/pubmed/17545527.

49. Tremblay MR, Lescarbeau A, Grogan MJ, Tan E, Lin G, Austad BC, et al. Discovery of a potent and orally active hedgehog pathway antagonist (IPI-926). J Med Chem. 2009; 52:4400–4418.

50. Sarangi A, Valadez JG, Rush S, Abel TW, Thompson RC, Cooper MK. Targeted inhibition of the Hedgehog pathway in established malignant glioma xenografts enhances survival. Oncogene. 2009; 28:3468–3476.

51. Nowicki MO, Pawlowski P, Fischer T, Hess G, Pawlowski T, Skorski T. Chronic myelogenous leukemia molecular signature. Oncogene. 2003; 22:3952–3963.

52. Liu XR, Cai Y, Cao X, Wei RC, Li HL, Zhou XM, et al. A new oncolytic adenoviral vector carrying dual tumour suppressor genes shows potent anti-tumour effect. J Cell Mol Med. 2012; 16:1298–1309.

53. Imai K, Hirata S, Irie A, Senju S, Ikuta Y, Yokomine K, et al. Identification of HLA-A2-restricted CTL epitopes of a novel tumour-associated antigen, KIF20A, overexpressed in pancreatic cancer. Br J Cancer. 2011; 104:300–307.

54. Ganguly A, Yang H, Cabral F. Overexpression of mitotic centromere-associated Kinesin stimulates microtubule detachment and confers resistance to paclitaxel. Mol Cancer Ther. 2011; 10:929–937.

55. Nakamura Y, Tanaka F, Haraguchi N, Mimori K, Matsumoto T, Inoue H, et al. Clinicopathological and biological significance of mitotic centromere-associated kinesin overexpression in human gastric cancer. Br J Cancer. 2007; 97:543–549.

56. Schimizzi GV, Currie JD, Rogers SL. Expression levels of a kinesin-13 microtubule depolymerase modulates the effectiveness of anti-microtubule agents. PLoS One. 2010; 5:e11381.

57. Grinberg-Rashi H, Ofek E, Perelman M, Skarda J, Yaron P, Hajdúch M, et al. The expression of three genes in primary non-small cell lung cancer is associated with metastatic spread to the brain. Clin Cancer Res. 2009; 15:1755–1761.

58. DeBonis S, Skoufias DA, Lebeau L, Lopez R, Robin G, Margolis RL, et al. In vitro screening for inhibitors of the human mitotic kinesin Eg5 with antimitotic and antitumor activities. Mol Cancer Ther. 2004; 3:1079–1090.
59. Sakowicz R, Finer JT, Beraud C, Crompton A, Lewis E, Fritsch A, et al. Antitumor activity of a kinesin inhibitor. Cancer Res. 2004; 64:3276–3280.

60. Luo L, Parrish CA, Nevins N, McNulty DE, Chaudhari AM, Carson JD, et al. ATP-competitive inhibitors of the mitotic kinesin KSP that function via an allosteric mechanism. Nat Chem Biol. 2007; 3:722–726.

61. Maliga Z, Kapoor TM, Mitchison TJ. Evidence that monastrol is an allosteric inhibitor of the mitotic kinesin Eg5. Chem Biol. 2002; 9:989–996.

62. El-Nassan HB. Advances in the discovery of kinesin spindle protein (Eg5) inhibitors as antitumor agents. Eur J Med Chem. 2013; 62:614–631.

63. Huszar D, Theoclitou ME, Skolnik J, Herbst R. Kinesin motor proteins as targets for cancer therapy. Cancer Metastasis Rev. 2009; 28:197–208.

64. Sarli V, Giannis A. Targeting the kinesin spindle protein: basic principles and clinical implications. Clin Cancer Res. 2008; 14:7583–7587.

65. Mayer TU, Kapoor TM, Haggarty SJ, King RW, Schreiber SL, Mitchison TJ. Small molecule inhibitor of mitotic spindle bipolarity identified in a phenotype-based screen. Science. 1999; 286:971–974.

66. Johnson RK, McCabe FL, Cauder E, Innlow L, Whitacre M, Winkler JD, et al. SB-715992, a potent and selective inhibitor of KSP mitotic kinesin, demonstrates broadspectrum activity in advanced murine tumors and human tumor xenografts. In : 93rd Annual Meeting of the American Association for Cancer Research; 2002 Apr 6-10; Moscone Convention Center, San Francisco (CA). Philadelphia (PA): American Association for Cancer Research;2002. Poster No. 1335.
67. Talapatra SK, Schüttelkopf AW, Kozielski F. The structure of the ternary Eg5-ADP-ispinesib complex. Acta Crystallogr D Biol Crystallogr. 2012; 68:1311–1319.

68. Cox CD, Coleman PJ, Breslin MJ, Whitman DB, Garbaccio RM, Fraley ME, et al. Kinesin spindle protein (KSP) inhibitors. 9. Discovery of (2S)-4-(2,5-difluorophenyl)-n-[(3R,4S)-3-fluoro-1-methylpiperidin-4-yl]-2-(h ydroxymethyl)-N-methyl-2-phenyl-2,5-dihydro-1H-pyrrol e-1-carboxamide (MK-0731) for the treatment of taxane-refractory cancer. J Med Chem. 2008; 51:4239–4252.

69. Jackson JR, Gilmartin A, Dhanak D, Knight S, Parrish C, Luo L, et al. A second generation KSP inhibitor, SB-743921, is a highly potent and active therapeutic in preclinical models of cancer. AACR Meet Abstr Online. 2006; 2006:B11.
70. Kim KH, Xie Y, Tytler EM, Woessner R, Mor G, Alvero AB. KSP inhibitor ARRY-520 as a substitute for Paclitaxel in Type I ovarian cancer cells. J Transl Med. 2009; 7:63.

71. Woessner R, Tunquist B, Lemieux C, Chlipala E, Jackinsky S, Dewolf W Jr, et al. ARRY-520, a novel KSP inhibitor with potent activity in hematological and taxane-resistant tumor models. Anticancer Res. 2009; 29:4373–4380.
72. Carter BZ, Mak DH, Woessner R, Gross S, Schober WD, Estrov Z, et al. Inhibition of KSP by ARRY-520 induces cell cycle block and cell death via the mitochondrial pathway in AML cells. Leukemia. 2009; 23:1755–1762.

73. Basso AD, Liu M, Dai C, Gray K, Nale L, Tevar S, et al. SCH 2047069, a novel oral kinesin spindle protein inhibitor, shows single-agent antitumor activity and enhances the efficacy of chemotherapeutics. Mol Cancer Ther. 2010; 9:2993–3002.

74. Hotha S, Yarrow JC, Yang JG, Garrett S, Renduchintala KV, Mayer TU, et al. HR22C16: a potent small-molecule probe for the dynamics of cell division. Angew Chem Int Ed Engl. 2003; 42:2379–2382.

75. Marcus AI, Peters U, Thomas SL, Garrett S, Zelnak A, Kapoor TM, et al. Mitotic kinesin inhibitors induce mitotic arrest and cell death in Taxol-resistant and -sensitive cancer cells. J Biol Chem. 2005; 280:11569–11577.

76. Hwang MK, Min YK, Kim SH. Kinesin spindle protein inhibitor HR22C16 sensitizes TRAIL-induced apoptosis in human lung cancer H1299 cells. Bull Korean Chem Soc. 2011; 32:1737–1740.

77. Yang L, Jiang C, Liu F, You QD, Wu WT. Cloning, enzyme characterization of recombinant human Eg5 and the development of a new inhibitor. Biol Pharm Bull. 2008; 31:1397–1402.

78. Nakai R, Iida S, Takahashi T, Tsujita T, Okamoto S, Takada C, et al. K858, a novel inhibitor of mitotic kinesin Eg5 and antitumor agent, induces cell death in cancer cells. Cancer Res. 2009; 69:3901–3909.

79. Jiang C, You Q. Kinesin spindle protein inhibitors in cancer: a patent review (2008 - present). Expert Opin Ther Pat. 2013; 23:1547–1560.

80. Jiang C, Yang L, Wu WT, Guo QL, You QD. CPUYJ039, a newly synthesized benzimidazole-based compound, is proved to be a novel inducer of apoptosis in HCT116 cells with potent KSP inhibitory activity. J Pharm Pharmacol. 2011; 63:1462–1469.

81. Chen LC, Rosen LS, Iyengar T, Goldman JW, Savage R, Kazakin J, et al. First in human study with ARQ 621, a novel inhibitor of Eg5: final results. J Clin Oncol (Meeting Abstracts). 2011; 29:3076.
82. Shimizu M, Ishii H, Ogo N, Unno Y, Matsuno K, Sawada J, et al. S-trityl-L-cysteine derivative induces caspase-independent cell death in K562 human chronic myeloid leukemia cell line. Cancer Lett. 2010; 298:99–106.

83. Ogo N, Oishi S, Matsuno K, Sawada J, Fujii N, Asai A. Synthesis and biological evaluation of L-cysteine derivatives as mitotic kinesin Eg5 inhibitors. Bioorg Med Chem Lett. 2007; 17:3921–3924.

84. Hayashi N, Koller E, Fazli L, Gleave ME. Effects of Eg5 knockdown on human prostate cancer xenograft growth and chemosensitivity. Prostate. 2008; 68:1283–1295.

85. Liu M, Yu H, Huo L, Liu J, Li M, Zhou J. Validating the mitotic kinesin Eg5 as a therapeutic target in pancreatic cancer cells and tumor xenografts using a specific inhibitor. Biochem Pharmacol. 2008; 76:169–178.

86. Leizerman I, Avunie-Masala R, Elkabets M, Fich A, Gheber L. Differential effects of monastrol in two human cell lines. Cell Mol Life Sci. 2004; 61:2060–2070.

87. Vijapurkar U, Wang W, Herbst R. Potentiation of kinesin spindle protein inhibitor-induced cell death by modulation of mitochondrial and death receptor apoptotic pathways. Cancer Res. 2007; 67:237–245.

88. Shi J, Orth JD, Mitchison T. Cell type variation in responses to antimitotic drugs that target microtubules and kinesin-5. Cancer Res. 2008; 68:3269–3276.

89. Liu M, Aneja R, Liu C, Sun L, Gao J, Wang H, et al. Inhibition of the mitotic kinesin Eg5 up-regulates Hsp70 through the phosphatidylinositol 3-kinase/Akt pathway in multiple myeloma cells. J Biol Chem. 2006; 281:18090–18097.

90. Tunquist BJ, Woessner RD, Walker DH. Mcl-1 stability determines mitotic cell fate of human multiple myeloma tumor cells treated with the kinesin spindle protein inhibitor ARRY-520. Mol Cancer Ther. 2010; 9:2046–2056.

91. Tang Y, Orth JD, Xie T, Mitchison TJ. Rapid induction of apoptosis during Kinesin-5 inhibitor-induced mitotic arrest in HL60 cells. Cancer Lett. 2011; 310:15–24.

92. Kashina AS, Baskin RJ, Cole DG, Wedaman KP, Saxton WM, Scholey JM. A bipolar kinesin. Nature. 1996; 379:270–272.

93. Orth JD, Loewer A, Lahav G, Mitchison TJ. Prolonged mitotic arrest triggers partial activation of apoptosis, resulting in DNA damage and p53 induction. Mol Biol Cell. 2012; 23:567–576.

94. Yin Y, Sun H, Xu J, Xiao F, Wang H, Yang Y, et al. Kinesin spindle protein inhibitor SB743921 induces mitotic arrest and apoptosis and overcomes imatinib resistance of chronic myeloid leukemia cells. Leuk Lymphoma. 2014; 1–8.

95. Burris HA, Lorusso P, Jones S, Guthrie TM, Orr JB, Williams DD, et al. Phase I trial of novel kinesin spindle protein (KSP) inhibitor SB-715992 IV days 1, 8, 15 q 28 days. J Clin Oncol (Meeting Abstracts). 2004; 22:2004.

96. Heath EI, Alousi A, Eder JP, Valdivieso M, Vasist LS, Appleman L, et al. A phase I dose escalation trial of ispinesib (SB-715992) administered days 1-3 of a 21-day cycle in patients with advanced solid tumors. J Clin Oncol (Meeting Abstracts). 2006; 24:2026.

97. Chu QS, Holen KD, Rowinsky EK, Wilding G, Volkman JL, Orr JB, et al. Phase I trial of novel kinesin spindle protein (KSP) inhibitor SB-715992 IV Q 21 days. J Clin Oncol (Meeting Abstracts). 2004; 22:2078.

98. Miller K, Ng C, Ang P, Brufsky AM, Lee SC, Dees EC, et al. Abstract 1089. Phase II, open label study of SB-715992 (Ispinesib) in subjects with advanced or metastatic breast cancer. Breast Cancer Res Treat. 2005; 94:S70.
99. Tang PA, Siu LL, Chen EX, Hotte SJ, Chia S, Schwarz JK, et al. Phase II study of ispinesib in recurrent or metastatic squamous cell carcinoma of the head and neck. Invest New Drugs. 2008; 26:257–264.

100. Beer TM, Goldman B, Synold TW, Ryan CW, Vasist LS, Van Veldhuizen PJ Jr, et al. Southwest Oncology Group phase II study of ispinesib in androgen-independent prostate cancer previously treated with taxanes. Clin Genitourin Cancer. 2008; 6:103–109.

101. Purcell JW, Davis J, Reddy M, Martin S, Samayoa K, Vo H, et al. Activity of the kinesin spindle protein inhibitor ispinesib (SB-715992) in models of breast cancer. Clin Cancer Res. 2010; 16:566–576.

102. Carol H, Lock R, Houghton PJ, Morton CL, Kolb EA, Gorlick R, et al. Initial testing (stage 1) of the kinesin spindle protein inhibitor ispinesib by the pediatric preclinical testing program. Pediatr Blood Cancer. 2009; 53:1255–1263.

103. Souid AK, Dubowy RL, Ingle AM, Conlan MG, Sun J, Blaney SM, et al. A pediatric phase I trial and pharmacokinetic study of ispinesib: a Children's Oncology Group phase I consortium study. Pediatr Blood Cancer. 2010; 55:1323–1328.

104. Knox JJ, Gill S, Synold TW, Biagi JJ, Major P, Feld R, et al. A phase II and pharmacokinetic study of SB-715992, in patients with metastatic hepatocellular carcinoma: a study of the National Cancer Institute of Canada Clinical Trials Group (NCIC CTG IND.168). Invest New Drugs. 2008; 26:265–272.

105. Lee CW, Bélanger K, Rao SC, Petrella TM, Tozer RG, Wood L, et al. A phase II study of ispinesib (SB-715992) in patients with metastatic or recurrent malignant melanoma: a National Cancer Institute of Canada Clinical Trials Group trial. Invest New Drugs. 2008; 26:249–255.

106. Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005; 5:275–284.

107. Mimeault M, Hauke R, Batra SK. Recent advances on the molecular mechanisms involved in the drug resistance of cancer cells and novel targeting therapies. Clin Pharmacol Ther. 2008; 83:673–691.

108. Khoury HJ, Garcia-Manero G, Borthakur G, Kadia T, Foudray MC, Arellano M, et al. A phase 1 dose-escalation study of ARRY-520, a kinesin spindle protein inhibitor, in patients with advanced myeloid leukemias. Cancer. 2012; 118:3556–3564.

109. Kantarjian HM, Padmanabhan S, Stock W, Tallman MS, Curt GA, Li J, et al. Phase I/II multicenter study to assess the safety, tolerability, pharmacokinetics and pharmacodynamics of AZD4877 in patients with refractory acute myeloid leukemia. Invest New Drugs. 2012; 30:1107–1115.

110. Infante JR, Kurzrock R, Spratlin J, Burris HA, Eckhardt SG, Li J, et al. A Phase I study to assess the safety, tolerability, and pharmacokinetics of AZD4877, an intravenous Eg5 inhibitor in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2012; 69:165–172.

111. Holen K, DiPaola R, Liu G, Tan AR, Wilding G, Hsu K, et al. A phase I trial of MK-0731, a kinesin spindle protein (KSP) inhibitor, in patients with solid tumors. Invest New Drugs. 2012; 30:1088–1095.

112. Lee RT, Beekman KE, Hussain M, Davis NB, Clark JI, Thomas SP, et al. A university of Chicago consortium phase II trial of SB-715992 in advanced renal cell cancer. Clin Genitourin Cancer. 2008; 6:21–24.

113. Wakui H, Yamamoto N, Kitazono S, Mizugaki H, Nakamichi S, Fujiwara Y, et al. A phase 1 and dose-finding study of LY2523355 (litronesib), an Eg5 inhibitor, in Japanese patients with advanced solid tumors. Cancer Chemother Pharmacol. 2014; 74:15–23.

114. Shih KC, Infante JR, Papadopoulos KP, Bendell JC, Tolcher AW, Burris HA, et al. administered on Days 1, 2, and 3 with or without pegfilgrastim in patients with advanced malignancy (NCT01214629). J Clin Oncol (Meeting Abstracts). 2011; 29:2600.
115. Hentsch B. 4SC announces treatment of first patient in a phase I study of 4SC-205. Oral Eg5 kinesin spindle protein inhibitor to be evaluated in solid tumour and malignant lymphoma patients. Planegg-Martinsried: 4SC AG;2010.
116. Mross KB, Scharr D, Richly H, Frost A, Bauer S, Krauss B, et al. First in human study of 4SC-205 (AEGIS), a novel oral inhibitor of Eg5 kinesin spindle protein. J Clin Oncol (Meeting Abstracts). 2014; 32:2564.

117. Burris HA 3rd, Jones SF, Williams DD, Kathman SJ, Hodge JP, Pandite L, et al. A phase I study of ispinesib, a kinesin spindle protein inhibitor, administered weekly for three consecutive weeks of a 28-day cycle in patients with solid tumors. Invest New Drugs. 2011; 29:467–472.

118. Blagden SP, Molife LR, Seebaran A, Payne M, Reid AH, Protheroe AS, et al. A phase I trial of ispinesib, a kinesin spindle protein inhibitor, with docetaxel in patients with advanced solid tumours. Br J Cancer. 2008; 98:894–899.

119. Holen KD, Belani CP, Wilding G, Ramalingam S, Volkman JL, Ramanathan RK, et al. A first in human study of SB-743921, a kinesin spindle protein inhibitor, to determine pharmacokinetics, biologic effects and establish a recommended phase II dose. Cancer Chemother Pharmacol. 2011; 67:447–454.

120. Rosen L, Chen LC, Iyengar T, Goldman J, Lahr S, Chen CR, et al. Abstract 2750: ARQ 621, a novel potent and selective inhibitor of Eg5: preclinical data and early results from a clinical phase 1 study. Cancer Res. 2010; 70:2750.

122. Tan MH, De S, Bebek G, Orloff MS, Wesolowski R, Downs-Kelly E, et al. Specific kinesin expression profiles associated with taxane resistance in basal-like breast cancer. Breast Cancer Res Treat. 2012; 131:849–858.

123.
S Kim
. Protein structural biomarkers to guide targeted chemotherapies. United States patent US 20120122132 A1. 2012. 05. 17.
125. Gerecitano JF, Stephenson JJ, Lewis NL, Osmukhina A, Li J, Wu K, et al. A phase I trial of the kinesin spindle protein (Eg5) inhibitor AZD4877 in patients with solid and lymphoid malignancies. Invest New Drugs. 2013; 31:355–362.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download