Abstract
Increases in cardiovascular disease (CVD) in modern society are attributable to aging and lifestyle changes such as westernized diet and decreased physical activity. On the other hand, mounting evidence suggests that environmental pollutants such as persistent organic pollutants (POPs), bisphenol A (BPA) and phthalates are also related to the increases in CVD. POPs are a family of lipophilic stable chemicals that accumulate in adipose tissue and create a persistent toxic effect. The association between POPs and CVD is reported through epidemiologic, animal and in vitro studies. The association between BPA and CVD has also been established from many epidemiologic studies; however, a causal relationship remains uncertain. Exposure to POPs or BPA is also associated with the development of well-known CV risk factors such as type 2 diabetes mellitus, hypertension, hypercholesterolemia and obesity. Therefore, it is uncertain whether POPs and BPA are involved directly to the pathogenesis of atherosclerosis or indirectly associated with CVD. Additional longitudinal and experimental studies searching for the direct causal relationship and exact linking mechanisms should be conducted to investigate the effect of exposure to environmental pollutants such as POPs and BPA.
Figures and Tables
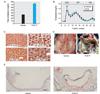 | Fig. 1Effects of PCB-77 on total serum cholesterol and VLDL cholesterol concentrations, lipid deposition within the liver and abdominal cavity, and atherosclerosis in ApoE-/- mice.24 (A) Total serum cholesterol concentrations (n=10 mice per group). Total serum cholesterol concentrations were markedly increased in ApoE-/- mice injected with PCB-77 compared with vehicle, (B) Lipoprotein cholesterol distributions (n=4 mice per group). Elevations in serum cholesterol concentrations in PCB-77-treated mice were predominantly in very low-density lipoprotein (VLDL) cholesterol, (C) Representative tissue sections from livers of mice injected with vehicle or PCB-77. Compared with vehicle-treated mice, tissue sections of liver from mice administered PCB-77 exhibited lipid-laden vacuoles, (D) Administration of PCB-77 resulted in marked deposition of lipid within the abdominal cavity, (E) Aortic root sections stained with oil red O from vehicle or PCB-77-injected mice. Administration of PCB-77 to ApoE-/- mice increased atherosclerosis in aortic root sections. *Significantly different from vehicle (p<0.05). |
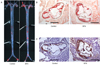 | Fig. 2Atherosclerotic lesions in the aorta and aortic sinus of ApoE-/- mice. (A) Representative photographs of aortas stained with Oil red O. Magnification ×40, (B) Representative photographs of aortic sinuses stained with Oil red O. Scale bars represent 1 mm, (C) Representative photographs of aortic sinuses stained with an anti-F4/80 antibody. Scale bars represent 1 mm. |
Table 1
Number of cases/total number and adjusted OR (95% CI) for prevalence of cardiovascular diseases by quartiles of PCDDs, PCDFs, dioxin-like PCBs, nondioxin-like PCBs, and OC pesticides in females.7

References
1. Statistics Korea. Cause of death statistics 2012. Daejeon: Statistics Korea;2013.
2. Bertazzi PA, Consonni D, Bachetti S, Rubagotti M, Baccarelli A, Zocchetti C, et al. Health effects of dioxin exposure: a 20-year mortality study. Am J Epidemiol. 2001; 153:1031–1044.

3. Bertazzi PA, Bernucci I, Brambilla G, Consonni D, Pesatori AC. The Seveso studies on early and long-term effects of dioxin exposure: a review. Environ Health Perspect. 1998; 106:Suppl 2. 625–633.

4. Hooiveld M, Heederik DJ, Kogevinas M, Boffetta P, Needham LL, Patterson DG Jr, et al. Second follow-up of a Dutch cohort occupationally exposed to phenoxy herbicides, chlorophenols, and contaminants. Am J Epidemiol. 1998; 147:891–901.

5. Pesatori AC, Zocchetti C, Guercilena S, Consonni D, Turrini D, Bertazzi PA. Dioxin exposure and non-malignant health effects: a mortality study. Occup Environ Med. 1998; 55:126–131.

6. Vena J, Boffetta P, Becher H, Benn T, Bueno-de-Mesquita HB, Coggon D, et al. Exposure to dioxin and nonneoplastic mortality in the expanded IARC international cohort study of phenoxy herbicide and chlorophenol production workers and sprayers. Environ Health Perspect. 1998; 106:Suppl 2. 645–653.

7. Ha MH, Lee DH, Jacobs DR. Association between serum concentrations of persistent organic pollutants and self-reported cardiovascular disease prevalence: results from the National Health and Nutrition Examination Survey, 1999-2002. Environ Health Perspect. 2007; 115:1204–1209.

8. Lang IA, Galloway TS, Scarlett A, Henley WE, Depledge M, Wallace RB, et al. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA. 2008; 300:1303–1310.

9. Melzer D, Osborne NJ, Henley WE, Cipelli R, Young A, Money C, et al. Urinary bisphenol A concentration and risk of future coronary artery disease in apparently healthy men and women. Circulation. 2012; 125:1482–1490.

10. Lind L, Lind PM. Can persistent organic pollutants and plastic-associated chemicals cause cardiovascular disease? J Intern Med. 2012; 271:537–553.

11. Van den Berg M, Birnbaum LS, Denison M, De Vito M, Farland W, Feeley M, et al. The 2005 World Health Organization reevaluation of human and Mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol Sci. 2006; 93:223–241.

12. Wang SL, Tsai PC, Yang CY, Guo YL. Increased risk of diabetes and polychlorinated biphenyls and dioxins: a 24-year follow-up study of the Yucheng cohort. Diabetes Care. 2008; 31:1574–1579.
13. Gustavsson P, Hogstedt C. A cohort study of Swedish capacitor manufacturing workers exposed to polychlorinated biphenyls (PCBs). Am J Ind Med. 1997; 32:234–239.

14. Calvert GM, Wall DK, Sweeney MH, Fingerhut MA. Evaluation of cardiovascular outcomes among U.S. workers exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Environ Health Perspect. 1998; 106:Suppl 2. 635–643.

15. Kang HK, Dalager NA, Needham LL, Patterson DG Jr, Lees PS, Yates K, et al. Health status of Army Chemical Corps Vietnam veterans who sprayed defoliant in Vietnam. Am J Ind Med. 2006; 49:875–884.

16. Sergeev AV, Carpenter DO. Hospitalization rates for coronary heart disease in relation to residence near areas contaminated with persistent organic pollutants and other pollutants. Environ Health Perspect. 2005; 113:756–761.

17. Lee DH, Lind L, Jacobs DR Jr, Salihovic S, van Bavel B, Lind PM. Associations of persistent organic pollutants with abdominal obesity in the elderly: the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) study. Environ Int. 2012; 40:170–178.

18. Brotons JA, Olea-Serrano MF, Villalobos M, Pedraza V, Olea N. Xenoestrogens released from lacquer coatings in food cans. Environ Health Perspect. 1995; 103:608–612.

19. Olea N, Pulgar R, Pérez P, Olea-Serrano F, Rivas A, Novillo-Fertrell A, et al. Estrogenicity of resin-based composites and sealants used in dentistry. Environ Health Perspect. 1996; 104:298–305.

20. Wang T, Li M, Chen B, Xu M, Xu Y, Huang Y, et al. Urinary bisphenol A (BPA) concentration associates with obesity and insulin resistance. J Clin Endocrinol Metab. 2012; 97:E223–E227.

21. Dalton TP, Kerzee JK, Wang B, Miller M, Dieter MZ, Lorenz JN, et al. Dioxin exposure is an environmental risk factor for ischemic heart disease. Cardiovasc Toxicol. 2001; 1:285–298.

22. Wu D, Nishimura N, Kuo V, Fiehn O, Shahbaz S, Van Winkle L, et al. Activation of aryl hydrocarbon receptor induces vascular inflammation and promotes atherosclerosis in apolipoprotein E-/- mice. Arterioscler Thromb Vasc Biol. 2011; 31:1260–1267.

23. Kopf PG, Huwe JK, Walker MK. Hypertension, cardiac hypertrophy, and impaired vascular relaxation induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin are associated with increased superoxide. Cardiovasc Toxicol. 2008; 8:181–193.

24. Arsenescu V, Arsenescu RI, King V, Swanson H, Cassis LA. Polychlorinated biphenyl-77 induces adipocyte differentiation and proinflammatory adipokines and promotes obesity and atherosclerosis. Environ Health Perspect. 2008; 116:761–768.

25. Arsenescu V, Arsenescu R, Parulkar M, Karounos M, Zhang X, Baker N, et al. Polychlorinated biphenyl 77 augments angiotensin II-induced atherosclerosis and abdominal aortic aneurysms in male apolipoprotein E deficient mice. Toxicol Appl Pharmacol. 2011; 257:148–154.

26. Lim S, Ahn SY, Song IC, Chung MH, Jang HC, Park KS, et al. Chronic exposure to the herbicide, atrazine, causes mitochondrial dysfunction and insulin resistance. PLoS One. 2009; 4:e5186.

27. Rogers JM, Ellis-Hutchings RG, Grey BE, Zucker RM, Norwood J Jr, Grace CE, et al. Elevated blood pressure in offspring of rats exposed to diverse chemicals during pregnancy. Toxicol Sci. 2014; 137:436–446.

28. Kim MJ, Moon MK, Kang GH, Lee KJ, Choi SH, Lim S, et al. Chronic exposure to bisphenol A can accelerate atherosclerosis in high-fat-fed apolipoprotein E knockout mice. Cardiovasc Toxicol. Forthcoming 2013.

29. Wei J, Lin Y, Li Y, Ying C, Chen J, Song L, et al. Perinatal exposure to bisphenol A at reference dose predisposes offspring to metabolic syndrome in adult rats on a high-fat diet. Endocrinology. 2011; 152:3049–3061.

30. Ryan KK, Haller AM, Sorrell JE, Woods SC, Jandacek RJ, Seeley RJ. Perinatal exposure to bisphenol-a and the development of metabolic syndrome in CD-1 mice. Endocrinology. 2010; 151:2603–2612.

31. Alonso-Magdalena P, Ropero AB, Soriano S, García-Arévalo M, Ripoll C, Fuentes E, et al. Bisphenol-A acts as a potent estrogen via non-classical estrogen triggered pathways. Mol Cell Endocrinol. 2012; 355:201–207.

32. Nadal A, Ropero AB, Laribi O, Maillet M, Fuentes E, Soria B. Nongenomic actions of estrogens and xenoestrogens by binding at a plasma membrane receptor unrelated to estrogen receptor alpha and estrogen receptor beta. Proc Natl Acad Sci U S A. 2000; 97:11603–11608.

33. Alonso-Magdalena P, Morimoto S, Ripoll C, Fuentes E, Nadal A. The estrogenic effect of bisphenol A disrupts pancreatic beta-cell function in vivo and induces insulin resistance. Environ Health Perspect. 2006; 114:106–112.

34. Hugo ER, Brandebourg TD, Woo JG, Loftus J, Alexander JW, Ben-Jonathan N. Bisphenol A at environmentally relevant doses inhibits adiponectin release from human adipose tissue explants and adipocytes. Environ Health Perspect. 2008; 116:1642–1647.

35. Kidani T, Kamei S, Miyawaki J, Aizawa J, Sakayama K, Masuno H. Bisphenol A downregulates Akt signaling and inhibits adiponectin production and secretion in 3T3-L1 adipocytes. J Atheroscler Thromb. 2010; 17:834–843.

36. Rubin BS, Murray MK, Damassa DA, King JC, Soto AM. Perinatal exposure to low doses of bisphenol A affects body weight, patterns of estrous cyclicity, and plasma LH levels. Environ Health Perspect. 2001; 109:675–680.

37. Rubin BS, Soto AM. Bisphenol A: perinatal exposure and body weight. Mol Cell Endocrinol. 2009; 304:55–62.

38. Howdeshell KL, Hotchkiss AK, Thayer KA, Vandenbergh JG, vom Saal FS. Exposure to bisphenol A advances puberty. Nature. 1999; 401:763–764.

39. Somm E, Schwitzgebel VM, Toulotte A, Cederroth CR, Combescure C, Nef S, et al. Perinatal exposure to bisphenol a alters early adipogenesis in the rat. Environ Health Perspect. 2009; 117:1549–1555.

40. Moon MK, Kim MJ, Jung IK, Koo YD, Ann HY, Lee KJ, et al. Bisphenol A impairs mitochondrial function in the liver at doses below the no observed adverse effect level. J Korean Med Sci. 2012; 27:644–652.

41. Nakagawa Y, Tayama S. Metabolism and cytotoxicity of bisphenol A and other bisphenols in isolated rat hepatocytes. Arch Toxicol. 2000; 74:99–105.

42. Bindhumol V, Chitra KC, Mathur PP. Bisphenol A induces reactive oxygen species generation in the liver of male rats. Toxicology. 2003; 188:117–124.

43. Asahi J, Kamo H, Baba R, Doi Y, Yamashita A, Murakami D, et al. Bisphenol A induces endoplasmic reticulum stress-associated apoptosis in mouse non-parenchymal hepatocytes. Life Sci. 2010; 87:431–438.

44. Shertzer HG, Nebert DW, Puga A, Ary M, Sonntag D, Dixon K, et al. Dioxin causes a sustained oxidative stress response in the mouse. Biochem Biophys Res Commun. 1998; 253:44–48.

45. Puga A, Hoffer A, Zhou S, Bohm JM, Leikauf GD, Shertzer HG. Sustained increase in intracellular free calcium and activation of cyclooxygenase-2 expression in mouse hepatoma cells treated with dioxin. Biochem Pharmacol. 1997; 54:1287–1296.

46. Sadhu DN, Merchant M, Safe SH, Ramos KS. Modulation of protooncogene expression in rat aortic smooth muscle cells by benzo[a]pyrene. Arch Biochem Biophys. 1993; 300:124–131.

47. Nebert DW, Roe AL, Dieter MZ, Solis WA, Yang Y, Dalton TP. Role of the aromatic hydrocarbon receptor and [Ah] gene battery in the oxidative stress response, cell cycle control, and apoptosis. Biochem Pharmacol. 2000; 59:65–85.

48. Kim KY, Kim DS, Lee SK, Lee IK, Kang JH, Chang YS, et al. Association of low-dose exposure to persistent organic pollutants with global DNA hypomethylation in healthy Koreans. Environ Health Perspect. 2010; 118:370–374.

49. Thackaberry EA, Jiang Z, Johnson CD, Ramos KS, Walker MK. Toxicogenomic profile of 2,3,7,8-tetrachlorodibenzo-p-dioxin in the murine fetal heart: modulation of cell cycle and extracellular matrix genes. Toxicol Sci. 2005; 88:231–241.

50. Murk AJ, Legler J, Denison MS, Giesy JP, van de Guchte C, Brouwer A. Chemical-activated luciferase gene expression (CALUX): a novel in vitro bioassay for Ah receptor active compounds in sediments and pore water. Fundam Appl Toxicol. 1996; 33:149–160.

51. Joung KE, Chung YH, Sheen YY. DRE-CALUX bioassay in comparison with HRGC/MS for measurement of toxic equivalence in environmental samples. Sci Total Environ. 2007; 372:657–667.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download