Abstract
Introduction
Transcatheter aortic valve implantation (TAVI) is now considered as an alternative treatment option for severe aortic stenosis (AS) patients who cannot undergo surgical aortic valve replacement (AVR).
Case report
We describe the first Korean case of transaortic TAVI with mini-sternotomy using CoreValve. A 83-year-old woman with severe AS and recent history of non-ST elevation myocardial infarction was referred to our institution for TAVI intervention. There was no amenable peripheral vascular access for transfemoral or trans-subclavian approach. Considering the relatively high procedural risk of transapical approach in this patient, we performed transaortic TAVI with mini-sternotomy.
Surgical aortic valve replacement (AVR) is the treatment of choice of symptomatic severe aortic stenosis (AS). With technological advancements, an alternative to surgical AVR, transcatheter aortic valve implantation (TAVI) has recently emerged and has been performed widely after the first case a decade ago.1,2 The most common route used to deliver transcatheter aortic valve is femoral artery. For patients with significant peripheral arterial diseases, especially common femoral or iliac artery stenosis, other access approach such as transapical or trans-subclavian approach can be considered. For whom with unsuitable conditions for previously mentioned routes, direct transaortic access with a mini-sternotomy could be an alternative option. In this case report, we describe the first Korean case of trans-aortic TAVI using CoreValve (Medtronic) for an 83-year-old woman.
An 83-year-old woman (height, 1.43 m; weight, 40 kg; body mass index, 19.6 kg/m2) with severe AS was referred to our institution with recent history of non-ST-elevation myocardial infarction. Transthoracic echocardiography indicated a heavily calcified aortic valve with normal global left ventricular ejection fraction of 63% (Fig. 1A). An aortic valve area was 0.6 cm2 by continuity equation. The peak and mean aortic pressure gradient was 97 mmHg and 63 mmHg, respectively (Fig. 1B). On computed tomography (CT), the mean diameter of the aortic annulus 19 mm and the perimeter was 60 mm. The diameter of ascending aorta was 30 mm. Coronary angiography showed one-vessel coronary artery disease with a focal stenosis (50% diameter stenosis) at the proximal right coronary artery. The patient was considered high risk for conventional open heart surgical AVR based on the logistic European System for Cardiac Operative Risk Evaluation (EuroSCORE) which was calculated to be 25.93%. On CT angiography, the maximal lumen diameter was 5 mm for both common femoral arteries and 6 mm for both iliac arteries (Fig. 2). The diameter of the left subclavian artery was 5.5 mm.
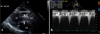
A multidisciplinary heart team consisted of interventional cardiologists, cardiac surgeons, cardiac imaging specialists and anesthesiologists reviewed the patient and decided to perform TAVI. Since a delivery catheter of the CoreValve (18 french (F)) requires a vascular route of ≥7 mm, transfemoral or trans-subclavian route was not eligible for TAVI. Transapical approach is not generally feasible with CoreValve system. Therefore, we planned to perform transaortic TAVI by a mini-sternotomy. The procedure was prepared in a hybrid operating room under the general anesthesia. A 5F pigtail catheter was introduced through the femoral artery and placed in the ascending aorta. Aortogram revealed a sufficient length (>7 cm) of ascending aorta. Since the CoreValve itself is 5 cm long, the distance from the aortic valve to the sheath's tip needs to be at least 6 cm in order to deploy the self-expandble CoreValve safely. For rapid ventricular pacing during balloon dilation of the aortic valve, a 4F balloon flotation temporary pacemaker wire was placed in the right ventricle via the femoral vein. After mini-sternotomy, ascending aorta was exposed (Fig. 3A) and a purse string sutures were made for the insertion of a 6F vascular sheath. The stenosed aortic valve was crossed with a 0.032 inch guidewire passed supported by a 5F Amplatz Left (AL) 1 catheter (Cordis, USA). The AL1 catheter was exchanged for pig-tail cathether. An Amplatz super stiff (260 cm, Amplatz Cook, Indiana, USA) wire was inserted over the pigtail and placed into the left ventricle. Thereafter, the 6F vascular sheath was exchanged for a 18F sheath (Fig. 3B). After the aortic valve was predilated with a 18 mm balloon (Z-med, NuMED Inc., Hopkinton, NY, USA) under rapid pacing, a 26 mm CoreValve was successfully deployed under fluoroscopic guidance (Fig. 3C, D). Aortography immediately after the CoreValve deployment demonstrated mild aortic regurgitation (grade II). Transesophageal echocardiography showed proper position of the prosthetic valve and confirmed mild paravalvular leak. The peak and mean transaortic pressure gradient was lowered to 17 mmHg and 8 mmHg, respectively (Fig. 4). The patient was monitored in a coronary care unit during 3 subsequent days for potential complications such as stroke, complete atrioventricular block, or vascular events. There was no significant interval change in electrocardiogram (ECG) findings between before the procedure and after that (Fig. 5). She recovered fully without any adverse event and was discharged on the 9th day after the procedure. The patient is currently being followed for 18 months without significant adverse cardiovascular event.
TAVI is accepted as an alternative treatment modality for patient with severe AS at high surgical risk. Patients with an estimated morality risk >20% by logistic EuroSCORE or >10% by Society of Thoracic Surgeons (STS) score system are generally considered candidates for the TAVI procedure. In the literature, TAVI and surgical AVR were associated with similar rates of survival at 1 year, although all neurologic events (including major strokes and transient ischemic attacks) and vascular complication after procedures were more associated with TAVI.2 Currently two types of stented valves, balloon-expandable Edwards SAPIEN valve and self-expandable CoreValve systems, are commonly used for TAVI.
Commonly, TAVI procedure is performed via femoral artery because its diameter is generally more than 6 mm so that 18F device introducer sheath and delivery catheter can be introduced. Small size common femoral or iliac arteries are contraindicated for a transfemoral approach. There is an increased risk of iliac rupture which can result in fatality, when forceful delivery of a large sheath attempted in small iliac arteries. In such cases, transaortic or trans-subclavian approach is generally recommended. Thus, when bilateral iliofemoral arteries are not eligible due to concomitant peripheral artery disease, alternative routes such as trans-subclavian, transapical, or direct aortic access through mini-thoracotomy/sternotomy could be considered. In our case, trans-subclavian approach was not amenable due to small diameter of subclavian artery.
Transapical approach is not feasible with CoreValve system. Furthermore, transapical approach is known to associated with the mortality from LV apical tear or rupture at the site of puncture,3 and has also complications including LV apical false aneurysm, arrhythmias, and echocardiographic hypokinesia or akinesia of the LV apex.4-6 With recent experiences, there were no significant differences in 30 day mortality or procedural complications between transapical approach and transaortic approach.7 In another study, potential advantages including ease of access and procedure related complication (e.g. false aneurysm, wound pain, respiratory dysfunction) compared with transapical approach were suggested.8
Direct aortic access can be obtained by mini-sternotomy as well as with a right anterior mini-thoracotomy.9 The direct aortic approach has advantage of overcoming challenging vascular disease, and avoids the risk of dislodging atherosclerotic plaque during procedure through the aorta that may cause distal embolism and subsequent stroke.9 Cannula access is technically easy and hemorrhage or late pseudoaneurysm risk is relatively low.8 Our patient tolerated wound pain very well and showed early respiratory and physical recovery without any adverse event. To our knowledge, this was the first case of successful transaortic TAVI in Korea.
In conclusion, an 83-year-old female patient without proper femoral or subclavian artery access successfully underwent trans-aortic TAVI via mini-sternotomy. This case suggests transaortic approach may be an effective and safe strategy for TAVI in high risk severe AS patients without eligible femoral or subclavian access routes.
Figures and Tables
Fig. 1
Transthoracic echocardiography before the transcatheter aortic valve implantation. (A) Parasternal long axis view shows heavily calcified stenotic aortic valve, (B) Pressure gradient over aortic valve by Doppler (maximal pressure gradient 97 mmHg, mean pressure gradient 63 mmHg). AV; aortic valve, Ao; aorta, LV; left ventricle, LA; left atrium.
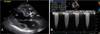
Fig. 2
CT images of aorta and iliofemoral arteries. Bilaterally, iliofemoral arteries were not eligible for the transcatheter aortic valve implantation route due to insufficient size (diameter <6 mm). CIA; common iliac artery, EIA; external iliac artery, CFA; common femoral artery.
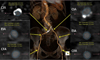
Fig. 3
Transcatheter aortic valve implantation by transaortic approach. (A) Exposure of ascending aorta by mini-sternotomy, (B) Insertion of a 18F sheath into ascending aorta, (C) Partial deployment of self-expanding CoreValve within aortic valve, (D) Complete implantation of CoreValve.
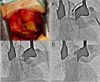
Fig. 4
Transthoracic echocardiography after the transcatheter aortic valve implantation. (A) Parasternal long axis view shows implanted CoreValve, (B) Pressure gradient over aortic valve by Doppler (maximal pressure gradient 17 mmHg, mean pressure gradient 8 mmHg). AV; aortic valve, Ao; aorta, LV; left ventricle, LA; left atrium.
References
1. Cribier A, Eltchaninoff H, Bash A, Borenstein N, Tron C, Bauer F, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation. 2002; 106:3006–3008.

2. Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, et al. Transcatheter versus surgical aorticvalve replacement in high-risk patients. N Engl J Med. 2011; 364:2187–2198.

3. Al-Attar N, Ghodbane W, Himbert D, Rau C, Raffoul R, Messika-Zeitoun D, et al. Unexpected complications of transapical aortic valve implantation. Ann Thorac Surg. 2009; 88:90–94.

4. Bleiziffer S, Ruge H, Mazzitelli D, Hutter A, Opitz A, Bauernschmitt R, et al. Survival after transapical and transfemoral aortic valve implantation: talking about two different patient populations. J Thorac Cardiovasc Surg. 2009; 138:1073–1080.

5. Ye J, Cheung A, Lichtenstein SV, Carere RG, Thompson CR, Pasupati S, et al. Transapical aortic valve implantation in humans. J Thorac Cardiovasc Surg. 2006; 131:1194–1196.

6. Elhenawy A, Rocha R, Feindel CM, Brister SJ. Persistent left ventricular false aneurysm after transapical insertion of an aortic valve. J Card Surg. 2011; 26:51–53.

7. Bapat V, Khawaja MZ, Attia R, Narayana A, Wilson K, Macgillivray K, et al. Transaortic Transcatheter Aortic valve implantation using Edwards Sapien valve: a novel approach. Catheter Cardiovasc Interv. 2012; 79:733–740.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download