Abstract
Resistant hypertension is defined as poorly controlled status of blood pressure despite of optimal use of three or more antihypertensive drugs of different classes, including diuretics. Although exact prevalence of resistant hypertension is not known, it has been reported to be 12.8% among patients treated with antihypertensive drugs. It is important to evaluate a possible secondary cause in patients with resistant hypertension. We report a case of resistant hypertension with renal artery segmental stenosis that was not revealed in renal Doppler study. Blood pressure of the patient was well controlled after renal balloon angioplasty.
Resistant hypertension is defined as poorly controlled status despite of optimal use of three antihypertensive drugs of different classes.1 Although exact prevalence of resistant hypertension is not known, it has been reported to be as high as 12.8% among patients treated with antihypertensive drugs.2 Recently, the incidence of resistant hypertension is increasing owing to the increase of the elderly population and obesity.1,3 As successful treatment require accurate diagnosis, it may be important to consider a secondary cause of hypertension in patient with resistant hypertension. We report a case of resistant hypertension patient with renal artery segmental stenosis, despite of three antihypertensive drugs of different classes.
A 45 year-old male who was diagnosed with hypertensive retinopathy due to left eye visual disturbance, visited an outpatient clinic for evaluation and treatment of hypertension. Despite escalating three different classes of antihypertensive medications (Losartan 50 mg, Hydrochlorothiazide 12.5 mg, Amlodipine besylate 5 mg) for 6 months, the maximal blood pressure was 210/110 mmHg during a follow-up in outpatient clinic. The 24 hour blood pressure monitoring showed a mean blood pressure of 145/86 mmHg and a maximal blood pressure of 175/110 mmHg. Finally, the patient was diagnosed with resistant hypertension, and the patient underwent study for secondary cause of hypertension.
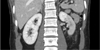
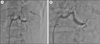
The past and family history of the patient were not remarkable and the body mass index of the patient was within normal limit (22.5 kg/m2). Physical examination including abdominal auscultation was not remarkable. The electrocardiogram and chest x-ray were within normal range and not remarkable. Laboratory study including electrolyte, urinary analysis, aldosterone/renin ratio, thyroid function test, blood urea nitrogen, and creatinine were within normal range. The norepinephrine was slightly elevated to 95.0 ug/day (15.0-80.0 ug/day) in the 24 hour urine study. However, the plasma metanephrine and normetanephrine were 0.32 mmol/L (0-0.5 mmol/L) and 0.68 mmol/L (0-0.9 mmol/L) respectively, which were both within normal range. The echocardiogram showed normal function of heart without evidence of coartation of the aorta. The patient underwent the renal doppler study for screening test of renovascular disease. The renal doppler was not significant, revealing right peak systolic velocity on proximal and distal portion were 123 cm/s and 126 cm/s and left peak systolic velocity on proximal and distal portion were 83 cm/s and 50 cm/s by renal duplex doppler ultrasonography. However, adrenal CT scan for elaborate evaluation of secondary hypertension showed decreased perfusion of mid and lower pole of left kidney due to possibility of renal artery segmental stenosis (Fig. 1). To evaluate for renal artery stenosis, the patient underwent renal angiography which revealed a severe segmental stenotic lesion in left renal artery (Fig. 2A). Balloon angioplasties were done on left renal artery segmental stenosis several times and the final angiogram showed optimal result of <30% residual stenosis without dissection (Fig. 2B). After balloon angioplasty of the left renal artery segmental stenosis, the followup 24 hour ambulatory blood pressure revealed mean blood pressure of 122/80 mmHg. During a follow-up 6 months in an outpatient clinic, blood pressure was well-controlled only with one medication (Amlodipine besylate 5 mg) and the patient was doing well without any symptoms.
Resistant hypertension is not an uncommon clinical problem. Risk factors of resistant hypertension are older age, obesity, chronic kidney disease, diabetes, longer hypertension duration, and presence of left ventricular hypertrophy.4 However, in a relatively young patient with sudden development of severe hypertension that is resistant to treatment, assessment of secondary cause should be performed.
Secondary hypertension is relatively common in patients with resistant hypertension.1 Some studies reported 12.7% of patients referred to a hypertension clinic center had a secondary cause of hypertension, although overall prevalence is unknown.5 Recent studies suggested that the patient with resistant hypertension should be considered for the secondary cause of hypertension.1,3,6 A common secondary cause of resistant hypertension are obstructive sleep apnea, renal parenchymal disease, primary aldosteronism, renal artery stenosis, and less common cause are pheochromatocytoma, cushing's syndrome. There are several clinical characteristics to suggest secondary hypertension. Clinical clues of secondary hypertension are young age less than 30 years, negative risk factor such as obesity, negative family history, and resistant hypertension. Among the causes of secondary hypertension, renal artery stenosis should be ruled out if there are clinical clues such as systolic-diastolic abdominal bruit, severe hypertension in patients with an unexplained atrophic kidney or deterioration of kidney function during antihypertensive therapy.7 However, since all the patients are not present with specific signs of renal artery stenosis, clinical suspicion is necessary in patients with resistant hypertension.
An important lesson from this case study is that renal artery segmental stenosis may be not detected on noninvasive imaging test including renal duplex doppler ultrasonography. The gold standard diagnostic tool is renal angiography for renal artery stenosis, but in real clinical practice, noninvasive test is preferred as initial diagnostic test.8 Renal duplex doppler ultrasonography with a sensitivity and specificity of 85% and 92% respectively is a common noninvasive tool as screening test for evaluation of renovascular disease.9 However its limitation is operator-dependency and the fact that it is less useful for evaluation of distal renal arteries.8 As the renal duplex doppler ultrasonography showed normal range of both renal artery peak systolic velocity, it was assumed there were no significant stenosis of both renal artery in this case.10 CT angiography (CTA) or Magnetic resonance angiography (MRA) are the most accurate, noninvasive diagnostic tools of renal artery stenosis.11 However, the accuracy of CT angiography and MR angiography for detecting distal segmental stenotic lesion has been noted only 64% for CTA and 62% for MRA respectively. As such, the risk of false negative result is more likely to happen in distal arterial segmental lesion such as fibromuscular dysplasia.12 Therefore, when results of noninvasive test are inconclusive, renal angiography should be considered in cases of high clinical suspicion.
Figures and Tables
References
1. Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, White A, Cushman WC, White W, Sica D, Ferdinand K, Giles TD, Falkner B, Carey RM. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension. 2008; 51:1403–1419.

2. Persell SD. Prevalence of resistant hypertension in the United States, 2003-2008. Hypertension. 2011; 57:1076–1080.

3. Sarafidis PA, Bakris GL. Resistant hypertension: an overview of evaluation and treatment. J Am Coll Cardiol. 2008; 52:1749–1757.
4. de la Sierra A, Banegas JR, Oliveras A, Gorostidi M, Segura J, de la Cruz JJ, Armario P, Ruilope LM. Clinical differences between resistant hypertensives and patients treated and controlled with three or less drugs. J Hypertens. 2012; 30:1211–1216.

6. Anderson GH Jr, Blakeman N, Streeten DH. The effect of age on prevalence of secondary forms of hypertension in 4429 consecutively referred patients. J Hypertens. 1994; 12:609–615.

7. Textor SC, Lerman L. Renovascular hypertension and ischemic nephropathy. Am J Hypertens. 2010; 23:1159–1169.

8. Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WR, Olin JW, Puschett JB, Rosenfield KA, Sacks D, Stanley JC, Taylor LM Jr, White CJ, White J, White RA, Antman EM, Smith SC Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. American Association for Vascular Surgery. Society for Vascular Surgery. Society for Cardiovascular Angiography and Interventions. Society for Vascular Medicine and Biology. Society of Interventional Radiology. ACC/AHA Task Force on Practice Guidelines Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease. American Association of Cardiovascular and Pulmonary Rehabilitation. National Heart, Lung, and Blood Institute. Society for Vascular Nursing. Trans-Atlantic Inter-Society Consensus. Vascular Disease Foundation. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients with Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006; 113:e463–e654.
9. Williams GJ, Macaskill P, Chan SF, Karplus TE, Yung W, Hodson EM, Craig JC. Comparative accuracy of renal duplex sonographic parameters in the diagnosis of renal artery stenosis: paired and unpaired analysis. AJR Am J Roentgenol. 2007; 188:798–811.

10. Chi YW, White CJ, Thornton S, Milani RV. Ultrasound velocity criteria for renal in-stent restenosis. J Vasc Surg. 2009; 50:119–123.

11. Glockner JF, Vrtiska TJ. Renal MR and CT angiography: current concepts. Abdom Imaging. 2007; 32:407–420.

12. Vasbinder GB, Nelemans PJ, Kessels AG, Kroon AA, Maki JH, Leiner T, Beek FJ, Korst MB, Flobbe K, de Haan MW, van Zwam WH, Postma CT, Hunink MG, de Leeuw PW, van Engelshoven JM. Renal Artery Diagnostic Imaging Study in Hypertension (RADISH) Study Group. Accuracy of computed tomographic angiography and magnetic resonance angiography for diagnosing renal artery stenosis. Ann Intern Med. 2004; 141:674–682.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download