Abstract
Stent thrombosis is a fatal complication that can cause sudden cardiac death in patients implanted coronary stent. Also, pulmonary thromboembolism is associated with increased mortality. Usually, these vascular thromboembolic diseases did not occured simultaneously. If this circumstance develops, possible mechanisms and causes should be described. Here, we report a case of patient underwent percutaneous coronary intervention under diagnosis of ST-segment elevation myocardial infarction with recurrent stent thrombosis and pulmonary thromboembolism associated with hyperhomocysteinemia despite optimal medical therapy.
Coronary stenting is an effective management in patient with acute myocardial infarction (AMI) with coronary stenosis or obstruction. However, stent thrombosis (ST) that can be developed after stenting is a fatal complication with higher mortality rates despite relative lower incidence.1 Also, pulmonary thromboembolism (PTE) is associated with increased mortality rates.2 These two vascular thromboembolic diseases usually did not occured simultaneously and have different pathophysiology, etiology, risk factors and management.
Here, we report a case of patient underwent percutaneous coronary intervention (PCI) under diagnosis of ST-segment elevation myocardial infarction with recurrent ST and PTE despite optimal medical therapy. Patient experienced all types of ST and concomitant PTE during follow up period. However, precipitating factors of these events were not detected except for hyperhomocysteinemia. We discussed the possible causes of recurrent ST with PTE and further therapeutic plans in these patients.
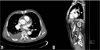
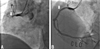
A 75-year-old male developed sudden chest pain 3 hours ago. Pain lasted during 1 hour and he suddenly collapsed. He was rushed unconscious to a neighborhood hospital by emergency medical services. And then he transferred to our hospital for further evaluation. He was a current smoker at 70 Pack-Years and no other atherosclerotic risk factors were presented. His initial rhythm was junctional bradycardia with 45 beats per minutes of heart rate and blood pressure was 70/40 mmHg. Also, 12-lead electrocardiogram showed ST-segment elevation in II, III, and aVF and V4R, V5R, V6R, V7, V8, and V9 leads on right posterior electrocardiogram. Primary PCI was performed and diagnostic coronary angiography showed thrombotic total occlusion in proximal right coronary artery (RCA) (Fig. 1A) and lesion was successfully revascularized with bare-metal stent (Coroflex-Blue 4.0×19 mm, B. Braun Systems) (Fig. 1B). 2-D echocardiography showed mild LV systolic dysfunction and hypokinesia in RCA territory. During admission, he complained chest discomfort and chest X-ray showed haziness in right lung. Therefore, chest computed tomography (CT) was checked. CT revealed filling defects in segmental pulmonary arteries in left lower lobe (Fig. 2A and 2B). Although RV dysfunction was presented, his vital sign and general condition was stable. We performed only anticoagulation without thrombolysis. He was discharged with triple ant-platelet therapy on event-free state 7 days later.
After 1 month, he revisited our emergency department with typical angina. 12-lead electrocardiogram showed T-inversion in II, III, and aVF. Urgent coronary angiography (CAG) showed thrombosis and de novo lesion in distal stent. We carried out direct stenting using bare-metal stent (Coroflex-Blue 3.5×19 mm, B. Braun Systems) (Fig. 3A to 3D). Despite of successful PCI, patient complained chest pain immediately after PCI. 12-lead ECG during development of pain showed STE in II, III, and aVF with complete atrioventricular block. Emergent CAG showed critical spasm over RCA and stent thrombosis (Fig. 4A). After nitrate and abciximab infusion, distal flow was improved (Fig. 4B). He did not have trouble with medication compliance and no chest pain was developed in other day except for the secondary admission day.
After discharge with other event, he revisited our hospital 5 months later. However, CAG at that time showed no in-stent restenosis, thrombosis and de novo lesions (Fig. 5A and 5B). He was discharge and followed up at outpatient department without angina. At 9 months after last admission, he revisited our emergency department with comatose mental status and hypotension. 12-lead ECG showed ST-segment elevation in II, III, and aVF with complete atrioventricular block. Emergent CAG revealed recurrent ST in proximal RCA (Fig. 6A) and we performed successful PCI with ballooning and final CAG showed TIMI (Thrombolysis In Myocardial Infarction) III antegrade flow with small amount of remnant thrombosis (Fig. 6B). During admission, no other cardiovascular events were developed and patient has been followed up at the outpatient department without any symptoms. In laboratory tests, platelet resistance test showed no resistance to anti-platelet (aspirin 449 ARU / P2Y12 90 PRU and 35%). Also, both leg venous CT angiogram showed no any filling defects and there were no abnormality in laboratory findings for evaluation of procoagulant state (antiphospholipid antibody, ANA, ANCA, EPO, factor V and VIII assay, lupus anticoagulant, protein C and S assay). But, hyperhomocysteinemia was detected in other tests for detection of thrombotic tendency (19.43 u mol/L; reference range 4.72 to 14.05 u mol/L). On genetic evaluation for hyperhomocysteinemia, the point mutation of methyltetrahydrofolate reductase (MTHFR) gene was not identified. So, we prescribed folate in addition to triple antiplatet therapy. Although he ceased warfarin ealier compared with recommended medication periods, other medication compliance was good (Fig. 7).
In interventional cardiology era, especially with coronary stenting, ST is a dreadful complication of PCI. It causes AMI or sudden cardiac death in the most cases despite its low incidence of 0.5% to 2.0% in bare metal stent (BMS).3 BMS implantation, rapid re-endothelization occurs within a few weeks after procedure contrary to drug-eluting stent (DES). Although recent several studies showed similar frequencies of ST episodes between DES and BMS, incidence of very late stent thrombosis (VLST) is still higher in DES than BMS.4 There are little studies or case report considering BMS ST, but a few case reports showed VLST in BMS or recurrent VLST in BMS.5 However, development of ST concomitant with PTE is very rare. Among risk factors of BMS ST such as noncompliance with antiplatelet agents, procoagulant state, history of coronary brachytherapy, small stent size, underdeployment of the stent, diabetes mellitus, renal failure, old age, and smoking, hyperhomocysteinemia and current smoking history can be applied to our patient.5 Because patient stopped smoking during follow-up periods, hyperhomocysteinemia might be associated with thrombotic tendency.
There has been controversy that hyperhomocysteinemia should be corrected in patients with vascular thromembolic disorders. Although experimental and clinical studies showed progression of atherosclerosis in hyperhomocysteinemia and relationship of homocysteine with coronary artery disease, homocysteine-lowering therapy using folate, vitamin B6 and B12 could not reduce risk of venous thromembolism.6,7 Also, our patient had only moderate elevation of serum homocysteine level and did not have the point mutation of methyltetrahydrofolate reductase (MTHFR) gene that is known to associated with progression of atherosclerosis.7 Relationship between hyperhomocysteinemia and PTE is also uncertain. In study 443 patients with venous thromboembolism including 219 patients with PTE and 304 matched controls, prevalence of hyperhomocysteinemia was found in all patients with no significant differences.8 However, Karalezli et al. reported that 46 patients with PTE showed increased level of homocysteine compared to health controls.9 Naturally, there is possibility that PTE and ST was occurred accidentally in our patient and further evaluation and observation is needed to find association PTE and ST. He suffered from recurrent angina and stent thrombosis (all types of stent thrombosis except for late stent thrombosis) despite ex-smoking, triple antiplatelet therapy and medications for coronary vasospasm. Although he ceased warfarin earlier due to non-compliance compared to recommend periods, there are no evidences that antiplatelet medication combined with oral anticoagulation could reduce the incidence of coronary stent thrombosis.10 Because there were no further correctable factors in this patient, we corrected hyperhomocysteinemia using folate. After then, he did not complain of any symptoms and follow-up CAG after 3-months since last ST episode showed no ST and in-stent restenosis (photo not shown).
In conclusion, homocysteine-lowering therapy such as folate might be considered to prevent vascular thrombotic events in hyperhomocysteinemic patients who suffered from recurrent vascular thromboembolic episodes without any correctable risk factors. Although there are no definitive evidences to support using folate, we think it is valuable in patient without other cardiovascular risk factors. It needs large scale randomized control trial to prove this issue.
Figures and Tables
Fig. 1
Primary PCI; CAG showed TTO in proximal RCA (A: white arrow) and successful PCI with coronary stenting (B: Coroflex-Blue 4.0×19 mm, B. Braun Systems).
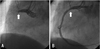
Fig. 3
Stent thrombosis and de novo lesion in distal to stent (A and B: arrow heads). Successful PCI with direct stenting using bare-metal stent (C and D: arrow heads, Coroflex-Blue 3.5×19 mm, B. Braun Systems).
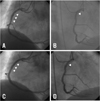
Fig. 4
Critical spasm over RCA and acute stent thrombosis (A: white arrow). Improved coronary flow after nitrate and abciximab infusion (B).
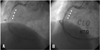
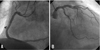
Fig. 5
Follow up coronary angiogram after 6 months until first admission day (A: Right coronary artery, B: Left coronary artery). There were no in-stent restenosis, stent thrombosis, and de novo lesion.
References
1. Cutlip DE, Baim DS, Ho KK, et al. Stent thrombosis in the modern era: a pooled analysis of multicenter coronary stent clinical trials. Circulation. 2001; 103:1967–1971.
2. Konstantinides S. Clinical practice. Acute pulmonary embolism. N Engl J Med. 2008; 359:2804–2813.
3. Park DW, Park SW. Stent thrombosis in the era of the drug-eluting stent. Korean Circ J. 2005; 35:791–794.

4. Jensen LO, Maeng M, Kaltoft A, et al. Stent thrombosis, myocardial infarction, and death after drug-eluting and bare-metal stent coronary interventions. J Am Coll Cardiol. 2007; 50:463–470.

5. Celik T, Iyisoy A, Celik M, Isik E. A case of very late stent thrombosis after bare metal coronary stent implantation: a neglected complication. Int J Cardiol. 2009; 134:111–113.

6. Lentz SR. Mechanisms of homocysteine-induced atherothrombosis. J Thromb Haemost. 2005; 3:1646–1654.

7. Den Heijer M, Lewington S, Clarke R. Homocysteine, MTHFR and risk of venous thrombosis: a meta-analysis of published epidemiological studies. J Thromb Haemost. 2005; 3:292–299.

8. Grifoni E, Marcucci R, Giutu G, et al. The thrombophilic pattern of different clinical manifestations of venous thromboembolism: a survey of 443 cases of venous thromboembolism. Semin thromb Hemost. 2012; 38:230–234.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download