Abstract
Purpose
Escherichia coli O157:H7 is one of the most important pathogens which create hemorrhagic colitis and hemolytic uremic syndrome in human. It is one of the most prevalent causes of diarrhea leading to death of many people every year. The first diagnosed gene in the locus of enterocyte effacement pathogenicity island is eae gene. The product of this gene is a binding protein called intimin belonging to the group of external membrane proteins regarded as a good stimulants of the immune system. Chitosan with its lipophilic property is an environmentally friendly agent able to return to the environment.
Materials and Methods
Intimin recombinant protein was expressed in pET28a vector with eae gene and purification was performed using Ni-NTA and finally the recombinant protein was approved through western blotting. This protein was encapsulated using chitosan nanoparticles and the size of nanoparticles was measured by Zetasizer. Intimin encapsulated was prescribed for three sessions among three groups of oral, injection, and oral-injection using Chitosan nanoparticles. Challenge was performed for all three groups with 108
E. coli O157:H7 bacteria.
Results
Intimin produced by chitosan nanoparticles improves immunological responses through the adjuvant nature of chitosan nanoparticles. Chitosan may be used as a carrier for transportation of the prescribed vaccine. Among the mice, encapsulated intimin could be able to provide suitable titers of IgG and IgA by the aid of chitosan nanoparticles. Results of mice challenge showed that decreased the bacterial shedding significantly.
Escherichia coli is a gram-negative cocobasilus belonging to Entrobacteriacca family. This microorganism is a natural intestinal flora of animals and humans which able to cause disease. E. coli is the most prevalent cause of urinary system infection such as pyelonephritis, cystitis and uretritis. Additionally E. coli can cause blood infection (sepsis), meningitis, and diarraha [12]. Sanitation principles is considered as one of the prevention procedures against infection in livestock. However, the drug resistance of pathogenic organisms against antibiotics is the main problem and cause eradicate of the natural flora [3]. Enterohemorrhagic serotype O157:H7 is regarded as the most prevalent cause of hemolytic uremic syndrome (HUS) [4]. After the attachment of the bacteria to the epithelial cells in intestine, the process of “attaching and effacing” (A/E) occurs and the shiga toxin is secreted into the blood stream. This toxin binds to tissues especially in kidney leading to HUS. Neutrophils are responsible for transporting shigatoxin in to blood stream and glumeroles [56]. Pilies help E. coli to bind with epithelial cells of the intestine. The most important pathogenic factor of enterohemorrhagic E. coli O157:H7 (EHEC) is its primary attachment with intestinal epithelial cells that leads to A/E process.
Since there is a bacteria clean up mechanism in intestinal epithelial cells, bacteria need special attachment system for passing through this barrier [78]. This attachment factor is encoded by eae chromosomal gene which called Intimin. Intimin is the first attachment factor of the bacterium to enterocyte then Tir protein through it transfer to host cell [9]. Intimin was introduced as a 94-kDa protein and was also proposed as the first genetic product related to the A/E pathologic process. Evidences showed that 650 amino acids of N-terminal of this protein are very similar to the antigenic protein in Yersinia pseudotuberculosis [10]. The first recognized gene in the locus of enterocyte effacement (LEE) pathogenicity island was called eae [11]. The eae gene is coded by polycistronic operon LEE5 and its product is a binding protein by the name of intimin [12]. Intimin is belonged to the outer membrane protein family produced by a group of entropatogenic and entrohemoragic E. coli [5].
The eae gene includes 2,805 nucleotide translated to 935 amino acids. Studies have shown that the intimin molecule can be classified as two distinct regions including (1) N-terminal amino acids and (2) carboxyl terminal amino acids [13]. The N-terminal region is conserved in all Intimin types but the carboxyl-terminal is completely variable in 280 terminal amino acids (Int280) which is responsible for binding with Tir protein. It is known as the heterogenecity factor for eae gene among different serotypes of Enterotoxigenic E. coli (ETEC) and Enterohemorrhagic E. coli (EHEC) [1415]. Studies indicated that all of intimin types have nearly similar G+C percent between 41.7 to 43.07 and their sequence length is also between 2,805 to 2,847 bp [16]. Local immunogenic responses provide a significant role in intestinal diseases. Mucosal antibodies are important factor for decreasing bacteria colonization. In this reason elevating specific antibody titer against antigens at the mucosal level will increase vaccine efficacy. Mucosal vaccination procedures such as oral or nasal routes are considered as effective and important ways for the induction of high antibody titers at the mucosal level [1718]. For such mucosal vaccines, lack of injection and sterilization process decreases the costs patients. However, these vaccines are not absorbed enough after mucosal immunization and need to adjuvants and encapsulation [1819]. The solution for this problem is to use nanoparticles for protein or antigen delivery. Because the related ingredient release slowly and the intercellular absorption rout such as the vesicular transportation is based on the M-cell in peyers patch. Nanoparticles are harvested from the surface of the M-cell and carried by lymphocytes in vesicular form and after that immunologic response and IgA production will begin [20]. Chitosan and its derivatives can attach to mucosa, increasing the drug and protein absorption when given orally. Studies have shown that microparticle and nanoparticle drugs loaded on chitosan will enhance drug absorption at the mucosal level. Chitosan and its derivatives temporarily open intercellular tight connections leading to intercellular transport of drugs and proteins [21].
The objective of the current study was to produce a nanovaccine against E. coli O157:H7 bacteria as a cause to diarrhea. In this study 280 amino acids from c-terminal of intimin were used. Recombinant intimin in E. coli was produced and loaded on chitosan nanoparticle. Finally, mice were administrated by chitosan containing the recombinant intimin and antibody titer was evaluated then challenge assay performed against E. coli O157:H7.
Chitosan with medium molecular weight, Sigma-Aldrich, (Germany), sodium tripoly phosphatase TPP (scharlau, Spain) were used. Chemical material, kits, and molecular markers from Merck (Germany), Sinagen (Iran), Qiagen (Germany) were also prepared.
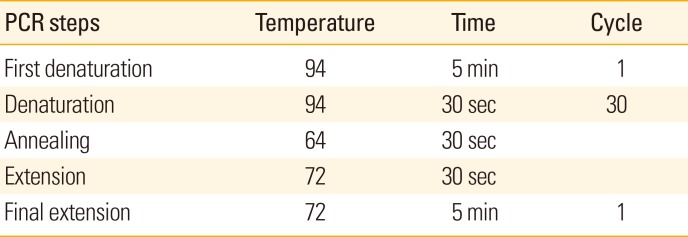
The Vector pET28a containing intimin gene was extracted by GeNetBio kit (Gobiz, korea) and polymerase chain reaction (PCR) was performed using specific primers. PCR was done based on the Table 1.
E. coli BL21DE3 containing pET28a-intimin was inoculated into 5-mL Luria-Bertani (LB) broth containing 80 µg/mL kanamycin located in 37℃ shaker incubator for 18 hours. Then, 100 µL of the grown bacteria was added to 5 mL LB broth containing 40 µg/mL kanamycin and then incubated in 37℃ shaker incubator until OD600 nm reached to 0.7. After that 1 mM IPTG was added to each tube and culture medium was incubated in shaker incubator (37℃, 150 rpm) to express the recombinant protein. Additionally, a non-inducted tube was used as control. After induction, 1 mL of test tube as well as the control was centrifuged (3,500 rpm for 5 minutes) and the gathered cells were mixed with 1×sample buffer and electrophoresed on 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gel.
Inoculated 2 mL of the E. coli BL21DE3 containing pET28a-intimin to 150 mL LB medium and incubated in shaker incubator for 3 hours (150 rpm) until OD600nm reached to 0.7 then IPTG (1 mM) was added. Because of presence of His-tag in pET28a vector, Ni-NTA column affinity chromatography was used for purification of recombinant protein. The pellet of E. coli BL21DE3 was solved in 100 mL denaturing buffer and sonication was done under 4℃. The suspension was centrifuged (13,000 rpm) for 20 minutes and gathered supernatant. First the column was equilibrated with buffer B (pH 8) and supernatant was loaded into column. The column was washed with washing buffer (pH 5.7). Then, the elution buffer (pH 4.5) was used and output column were gathered. In order to find the purity of the protein SDS-PAGE 12% was used. Bradford method was used for estimating the protein concentration [22].
One sample of recombinant bacteria containing pET28a-intimin inducted as well as the control sample (non-inducted) and the purified protein was electrophoresed on gel SDS-PAGE 12%. The membrane was cut based on the gel dimension and was put on the gel. The resulted sandwich was transfer in a tank with blotting buffer and running was carried on 45 minutes with the voltage of 75. After running the membrane was floated in the blocking buffer for 2 hours. Then, it washed with TBST buffer (TBS buffer+Tween 0.05%) three times. Antibody against the histidine terminal, 1:1,000 was prepared in TBST buffer and poured on nitrocellulose paper placed on shaker for one hour in 37℃ and then washed with TBST buffer three times each time for 10 minutes. DAB substrate tablet was dissolved in 5 mL deionized water and poured on nitrocellulose papers. The reaction was inhibited after the presence of bands [22].
In order to prepare chitosan sultion (2 mg/mL), 50 mg chitosan was dissolved in an final volume of 25 mL acetic acid 2% and for complete dissolving was placed on stirrer at the room temperature for one hour. Then, 50 mL of this soulution was added to 50 mL distilled water and put on the stirrer for one hour at the room temperature to be dissolved completely. The related antigen was added to 7.5 mL chitosan solution as drop by drop during a 10 minutes period until the final concentration of the antigen reached to 1 mg/mL. The solution PH was reached to 5.5 using NaOH in 30 minutes priod of time. After that, 5 mL sodium tripolyphosphate (TPP) was added to the chitosan antigen solution when stirring. At the end of reaction sonication was performed in a 20-second cycle. The chitosan without antigen was prepared as a control. Finally the solution was centrifuged in 13,000 rpm.
One hundred microliters of the supernatant was separated and the concentration of recombinant protein was evaluated and loading percentage of the antigen in nanoparticle was calculated.
The size of nanoparticles was evaluated by particle size analyser. The resulted sedimentation was separated in two parts then phosphate buffered saline (PBS) and deudinized water was added respectively.
To determine the amount of antigen release from nanoparticles, the relative protein concentration with Bradford test was used. Nanoparticles dissolved in PBS and deudinazed water were centrifuged for 120 minutes (14,000 rpm/4℃), and were put in shaker incubator for 6 hours (100 rpm/37℃). Sampling was performed from the supernatant each 90 minutes and Bradford test was performed for defining the antigen release from chitosan.
The 6 to 7 weeks female BALB/c mice were divided as three groups oral, oral-injection and injection. For the oral group, four times administration of nanoparticles containing intimin recmbinant protein (100 µg) was performed in the 2 weeks intervals. For the oral-injection group, three immunization of nanoparticles with recombinant protein (100 µg) was done and for final dose intimin recombinant protein (20 µg) was injected intrapretonealy. Forth groups, four step subcutaneous injection of intimin recombinant protein (20 µg) with Freund's Adjuvant Complete were implemented. For the injectional control group PBS was injected and for the oral control group chitosan without antigen was gavaged. Blood sampling from the eye corner was done ofter each injection or gavage procedure.
Enzyme-linked immunosorbent assay (ELISA) was done to determination IgG antibody titer in serum. ELISA plates were coated with 5 µg of recombinant intimin protein which was dissolved in coating buffer (Na2CO3, NaHCO3). The plate was then incubated in 4℃ for 16 hours. Then plate washed with PBST. After that the plate were incubated with 5% (W/V) bovine serum albumin (BSA) in PBST for 1 hour in 37℃ then washing with PBST, the mice control and test sera dilution (1:50 to 1:102400 for intentional group and 1:50 to 1:6,400 for oral group) were added to the ELISA plate and 1 hour incubated in 37℃ then the plate was washed and anti-mouse horseradish peroxidase (HRP) conjugated IgG was added to the wells and incubated for 1 hour in 37℃, the plate was washed and substrate was added to each well. Finally, the reaction was stopped by adding 100 µL of 2.5 M H2SO4 and the OD in 492 nm was read by ELISA reader.
For defining the IgA titer from 1:50 to 1:2,560 serum dilution was considered for three groups, and anti IgA HRP conjugate (1:1,000, Sigma) was used. The secretory IgA against the antigen in fecal samples was evaluated. In this procedure 0.5 g of fresh faeces was added to 1 mL PBS. After homogenization, the liquid supernatant was separated and from dilutions of 1:5 to 1:2,560 were evaluated by indirect ELISA.
Each mouse was gavaged with 109 of E. coli O157:H7 (ATCC: 35218). Fecal sampling was performed 2 days after gavage and repeated every 2 days. Fresh faeces samples were cultured on sorbitol MacConkey Agar at three different dilution (1:10,1:100, and 1:1,000) and white colonies were counted.
Each test represented of three independent experiments and showed as the mean±standard deviation (SD). Analyses were performed using a SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA). Multigroup comparisons were performed using two way ANOVA test. p<0.05 was considered significant.
To ensure the presence of intimin gene in pET28a plasmid, PCR was performed with T7 universal primers. The intimin fragment 1,245 bp was observed on 1% agarose gel (Fig. 1A).
The authenticity of recombinant intimin (35 kDa) proteins were confirmed by anti-poly His-tag antibody (Fig. 1D). In contrast, no reactivity was observed in negative control.
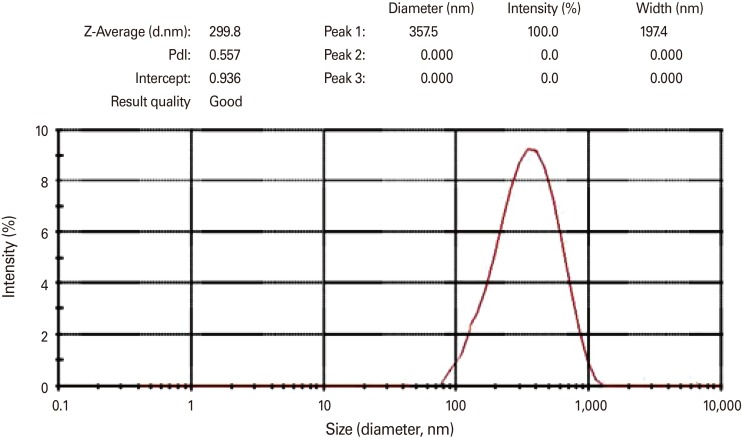
The potential zeta of nanoparticle is an important factor in the stability of the particles in solution. Chitosan and three methyl chitosan particles with zeta-positive potential have a greater stability in the solution. Moreover, higher zeta potential of chitosan lead to the adhesion particles to proteins and the mucosal surface and has a better ability to deliver protein in mucosal immune system (Fig. 2).
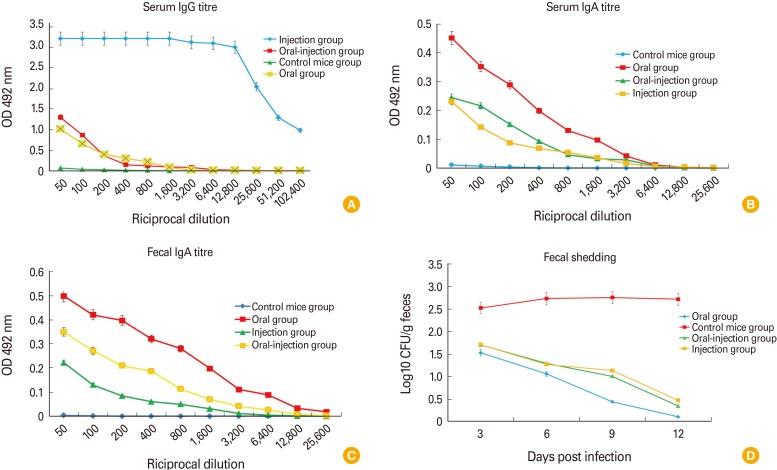
ELISA was used to determine the quantity the specific IgG and IgA antibodies against intimin protein. Results showed the specific IgG, serum IgA, and fecal IgA antibodies to intimin, respectively (Fig. 3A-C). These titration detected as early as the first immunization in the sera from immunized mice which increased significantly after the second booster. There was the significant difference (p<0.05) in antibody titers between intimin in the first injection. Antibody titres specific to intimin were not detected in the PBS controls. Some discrepancies were seen in the titres (end-point titre) which statistical analysis showed they were significant (p<0.05).
Fecal samples of three groups were collected two times per day for 2 weeks and cultured in sorbitol MacConkey agar. E. coli O157:H7 colonies were counted at the prepared dilution and results were plotted on a graph. Results indicated that in the oral group, the highest level of protection was observed in immunized mice. The error bar is standard deviation and differences were considered significant whenever p<0.05 (Fig. 3D).
Water and food contaminated with E. coli O157:H7 is the main cause of this bacterial infection among human. The main and most principal source of this bacterium are animals such as sheep and cattle. Enterohemorrhagic E. coli O157:H7 (EHEC) is the organism responsible for gastrointestinal infections ranging from simple diarrhea to severe entric inflammation with hemolytic uremic colitis and in some cases lead to HUS. During the infection, this bacterium is able to cause significant destructive lesions in the host enteric epithelial calls [23]. Economic and sanitary problems related to this bacterium is the reason for attention to infection with E. coli O157:H7. In livestock industry, this organism is the main cause of infection among young animals and currently, 15%–30% of these animals are involved with symptomatic and asymptomatic infection by this organism [324]. Death rates caused by diarrhea during the recent three decades shows that this disease is the second cause of childhood death below 5 years old worldwide [25]. Pathologically, this bacterium causes disease in two steps. It comes near to the host cells surface by its cellular organs such as fimbria and then attaches to the cells. This attachment disturbs the cell activity and producing some symptoms of A/E disease. In two steps, it secretes microbial toxins leading to more destructive impacts in nearby cells. These toxins are also transported to far away organ cells such as kidneys.
Prevention of this attachment process of the bacterium with the host cells or providing any disturbance in this attachment process may disrupt the occurrence of the other pathologic steps, caused by this bacterium. Through recognizing proteins involved in bacterial attachment process, effective vaccines will be planned and produced. Although many vaccines have been already planned against EHEC O157:H7 and experimented, but no effective vaccine for the prevention or control of such infection has been planned and entered the market globally [26]. For this reason, planning a vaccine against this bacterium, through recombinant protein technology and through the production of effective antigens is very essential. Many scientists have tried to produce effective vaccines against EHEC O157:H7 the following are mentioned. The first trial for planning such vaccine is related to late 1990th and first 2000th. In those years it was confirmed that secretary system proteins type III (TTSS), as well as an intramembrane protein (intimin), play a significant role on the attachment of EHEC bacterium to the cow's enteric cells [2728293031]. Crystallographic studies about intimin have shown that the carboxyl end of intimin protein is located at the outer side of the bacterial membrane and its main and middle parts are located in the cytoplasm and between the bilayer membrane. In fact, the carboxylic protein end can stimulate the immune system and it was shown that intimin is a very good candidate for the vaccine [10]. Studies have shown that intimin is the very potent immunogenic factor and in the serum of patients infected with E. coli O157:H7 the antibody against this molecule was found [3233]. A study conducted in 2015 is indicative of the effectiveness of intimin protein EspA and Tir. These proteins immunize cows against EHEC O157:H7 significantly. In the other study, Tir protein was eliminated and flagellin protein H7 was used instead. This procedure caused a high immunogenic activity. Investigation of colonies showed a significant decrease in fecal samples [343536]. In 2016 immunogenic reaction was studied among the mice vaccinated nasally. In this study recombinant protein EspB and 280 amino acids of C-terminal of intimin γ, as well as lipopeptide-2 was used for activating the immunity among the pregnant mice and results were evaluated. A group of mice was allowed to breastfeed their babies and another group was not allowed to do so. After the breastfeeding period, the serum of these mice was investigated, isolated immunization led to significant IgG titer among mice [37]. The main objective of this study was to prepare nanoparticles including recombinant intimin protein as a vaccine candidate and then the oral immunization of this nanoparticle to mice model in order to produce immunization through mucosal route. Two other immunization method (injection and oral-injection) of nanoparticles were also performed to define the most effective method for immunization. After gene induction, the recombinant protein was significantly expressed in insoluble phase in which the denaturing method and nickel column were used for purification. Refolding of this protein can produce antibody in animals and able to recognized and neutralized bacterial. In 2016, the potency of two nanoparticles as well as their immunization activity was investigated. Layered Double Hydroxides (LDH) and Hydroxyethyl cellulose nanoparticles two nanoparticles which have significant carriage ability of intimin B were used for mice immunization. Results were indicative of new generation of vaccine formulation able to control infectious diseases and LDH and HEC NPs are also able to induce the cellular immune response and strong antibodies [38].
Studies conducted about chitosan in 2016 showed that because of its ability to stimulate the cell-mediated immune system, chitosan is a good candidate for drug delivery and working as an adjuvant. Chitosan can increase the maturation of dendric cells. Through producing a type of interferon which was stimulated maturing the dendric cells [3940]. Physicochemical properties of nanoparticles including their types, sizes and immunogens loading percentages and protein release are among the effective factors of immunogenicity.
In this study, the size of nanoparticles was estimated as 299 nm and loading output was about 89.7% showing the good quality of nanoparticles and high protein loading output. Results showed that secretion IgA antibody titers in feces were higher than those in serum samples. Better results may be achieved through changing factors such as increasing the doses as well as improving the loading output and changing the nanoparticles. Results of ELISA study among three groups of oral, injection and oral-injection showed the higher titers of IgG among the injection group, followed by the oral-injection, and oral groups. For the IgA titers its level was highest in the oral group followed by the oral-injection and IgA titers in injection group was at the lowest range. The challenge on the mice showed less shedding rate among the oral groups than others indicative of higher protection rate in oral group. According to the results of this study, intimin included nano-vaccine may be regarded as a good candidate for protection against this bacteria.
References
1. Brooks GF, Jawetz E, Melnick JL, Adelberg EA. Jawetz, Melnick and Adelberg's medical microbiology. New York: McGraw Hill Medical;2010.
2. Murray PR, Rosenthal KS, Kobayashi GS, Pfaller MA. Medical microbiology. St. Louis, MO: Mosby;2002. p. 429–441.
3. Murphy BP, Murphy M, Buckley JF, et al. In-line milk filter analysis: Escherichia coli O157 surveillance of milk production holdings. Int J Hyg Environ Health. 2005; 208:407–413. PMID: 16217925.

4. Blanco JE, Blanco M, Alonso MP, et al. Serotypes, virulence genes, and intimin types of Shiga toxin (verotoxin)-producing Escherichia coli isolates from human patients: prevalence in Lugo, Spain, from 1992 through 1999. J Clin Microbiol. 2004; 42:311–319. PMID: 14715771.
5. te Loo DM, Monnens LA, van Der Velden TJ, et al. Binding and transfer of verocytotoxin by polymorphonuclear leukocytes in hemolytic uremic syndrome. Blood. 2000; 95:3396–3402. PMID: 10828021.

6. Chaisri U, Nagata M, Kurazono H, et al. Localization of Shiga toxins of enterohaemorrhagic Escherichia coli in kidneys of paediatric and geriatric patients with fatal haemolytic uraemic syndrome. Microb Pathog. 2001; 31:59–67. PMID: 11453701.

7. Kuhne SA, Hawes WS, La Ragione RM, Woodward MJ, Whitelam GC, Gough KC. Isolation of recombinant antibodies against EspA and intimin of Escherichia coli O157:H7. J Clin Microbiol. 2004; 42:2966–2976. PMID: 15243046.
8. Sussman M. Escherichia coli: mechanisms of virulence. New York: Cambridge University Press;1997.
9. van Diemen PM, Dziva F, Abu-Median A, et al. Subunit vaccines based on intimin and Efa-1 polypeptides induce humoral immunity in cattle but do not protect against intestinal colonisation by enterohaemorrhagic Escherichia coli O157:H7 or O26:H. Vet Immunol Immunopathol. 2007; 116:47–58. PMID: 17258324.

10. Ghaem-Maghami M, Simmons CP, Daniell S, et al. Intimin-specific immune responses prevent bacterial colonization by the attaching-effacing pathogen Citrobacter rodentium. Infect Immun. 2001; 69:5597–5605. PMID: 11500434.
11. Wales AD, Woodward MJ, Pearson GR. Attaching-effacing bacteria in animals. J Comp Pathol. 2005; 132:1–26. PMID: 15629476.

12. Bertin Y, Boukhors K, Livrelli V, Martin C. Localization of the insertion site and pathotype determination of the locus of enterocyte effacement of shiga toxin-producing Escherichia coli strains. Appl Environ Microbiol. 2004; 70:61–68. PMID: 14711626.
13. Donnenberg MS, Whittam TS. Pathogenesis and evolution of virulence in enteropathogenic and enterohemorrhagic Escherichia coli. J Clin Invest. 2001; 107:539–548. PMID: 11238553.

14. Thapar N, Sanderson IR. Diarrhoea in children: an interface between developing and developed countries. Lancet. 2004; 363:641–653. PMID: 14987892.

15. Krause G, Zimmermann S, Beutin L. Investigation of domestic animals and pets as a reservoir for intimin- (eae) gene positive Escherichia coli types. Vet Microbiol. 2005; 106:87–95. PMID: 15737477.

16. Adu-Bobie J, Frankel G, Bain C, et al. Detection of intimins alpha, beta, gamma, and delta, four intimin derivatives expressed by attaching and effacing microbial pathogens. J Clin Microbiol. 1998; 36:662–668. PMID: 9508292.
17. Svennerholm AM. From cholera to enterotoxigenic Escherichia coli (ETEC) vaccine development. Indian J Med Res. 2011; 133:188–196. PMID: 21415493.
18. Fujkuyama Y, Tokuhara D, Kataoka K, et al. Novel vaccine development strategies for inducing mucosal immunity. Expert Rev Vaccines. 2012; 11:367–379. PMID: 22380827.
19. Woodrow KA, Bennett KM, Lo DD. Mucosal vaccine design and delivery. Annu Rev Biomed Eng. 2012; 14:17–46. PMID: 22524387.

20. Hans ML, Lowman AM. Biodegradable nanoparticles for drug delivery and targeting. Curr Opin Solid State Mater Sci. 2002; 6:319–327.

21. Cho EJ, Holback H, Liu KC, Abouelmagd SA, Park J, Yeo Y. Nanoparticle characterization: state of the art, challenges, and emerging technologies. Mol Pharm. 2013; 10:2093–2110. PMID: 23461379.

22. Bollag DM, Rozycki MD, Edelstein SJ. Protein methods. 2nd ed. New York: Wiley-Liss;1996.
23. Aslani MM, Bouzari S. An epidemiological study on Verotoxin-producing Escherichia coli (VTEC) infection among population of northern region of Iran (Mazandaran and Golestan provinces). Eur J Epidemiol. 2003; 18:345–349. PMID: 12803375.

24. Potter AA, Klashinsky S, Li Y, et al. Decreased shedding of Escherichia coli O157:H7 by cattle following vaccination with type III secreted proteins. Vaccine. 2004; 22:362–369. PMID: 14670317.

25. Walker CL, Aryee MJ, Boschi-Pinto C, Black RE. Estimating diarrhea mortality among young children in low and middle income countries. PLoS One. 2012; 7:e29151. PMID: 22235266.

26. Shariati Mehr K, Mousavi SL, Rasooli I, Amani J, Rajabi M. A DNA vaccine against Escherichia coli O157:H7. Iran Biomed J. 2012; 16:133–139. PMID: 23023214.
27. van Diemen PM, Dziva F, Stevens MP, Wallis TS. Identification of enterohemorrhagic Escherichia coli O26:H- genes required for intestinal colonization in calves. Infect Immun. 2005; 73:1735–1743. PMID: 15731074.
28. Dziva F, van Diemen PM, Stevens MP, Smith AJ, Wallis TS. Identification of Escherichia coli O157 : H7 genes influencing colonization of the bovine gastrointestinal tract using signature-tagged mutagenesis. Microbiology. 2004; 150(Pt 11):3631–3645. PMID: 15528651.
29. Cornick NA, Booher SL, Moon HW. Intimin facilitates colonization by Escherichia coli O157:H7 in adult ruminants. Infect Immun. 2002; 70:2704–2707. PMID: 11953416.
30. Dean-Nystrom EA, Bosworth BT, Moon HW, O'Brien AD. Escherichia coli O157:H7 requires intimin for enteropathogenicity in calves. Infect Immun. 1998; 66:4560–4563. PMID: 9712821.
31. Dean-Nystrom EA, Bosworth BT, Moon HW. Pathogenesis of Escherichia coli O157:H7 in weaned calves. Adv Exp Med Biol. 1999; 473:173–177. PMID: 10659355.

32. Paton AW, Manning PA, Woodrow MC, Paton JC. Translocated intimin receptors (Tir) of Shiga-toxigenic Escherichia coli isolates belonging to serogroups O26, O111, and O157 react with sera from patients with hemolytic-uremic syndrome and exhibit marked sequence heterogeneity. Infect Immun. 1998; 66:5580–5586. PMID: 9784578.
33. Li Y, Frey E, Mackenzie AM, Finlay BB. Human response to Escherichia coli O157:H7 infection: antibodies to secreted virulence factors. Infect Immun. 2000; 68:5090–5095. PMID: 10948130.
34. McNeilly TN, Mitchell MC, Corbishley A, et al. Optimizing the protection of cattle against Escherichia coli O157:H7 colonization through immunization with different combinations of H7 flagellin, Tir, Intimin-531 or EspA. PLoS One. 2015; 10:e0128391. PMID: 26020530.

35. Amani J, Salmanian AH, Rafati S, Mousavi SL. Immunogenic properties of chimeric protein from espA, eae and tir genes of Escherichia coli O157:H7. Vaccine. 2010; 28:6923–6929. PMID: 20709010.

36. Amani J, Mousavi SL, Rafati S, Salmanian AH. Immunogenicity of a plant-derived edible chimeric EspA, Intimin and Tir of Escherichia coli O157:H7 in mice. Plant Sci. 2011; 180:620–627. PMID: 21421410.

37. Rabinovitz BC, Larzabal M, Vilte DA, Cataldi A, Mercado EC. The intranasal vaccination of pregnant dams with Intimin and EspB confers protection in neonatal mice from Escherichia coli (EHEC) O157:H7 infection. Vaccine. 2016; 34:2793–2797. PMID: 27129423.

38. Chen W, Zhang B, Mahony T, Gu W, Rolfe B, Xu ZP. Efficient and durable vaccine against intimin beta of diarrheagenic E. coli induced by clay nanoparticles. Small. 2016; 12:1627–1639. PMID: 27000499.
39. Carroll EC, Jin L, Mori A, et al. The vaccine adjuvant chitosan promotes cellular immunity via DNA sensor cGAS-STING-dependent induction of type I interferons. Immunity. 2016; 44:597–608. PMID: 26944200.

40. Doavi T, Mousavi SL, Kamali M, Amani J, Fasihi Ramandi M. Chitosan-based intranasal vaccine against Escherichia coli O157:H7. Iran Biomed J. 2016; 20:97–108. PMID: 26724233.
Fig. 1
Depiction of polymerase chain reaction (PCR) product, expression, purification and western blot confirmation of recombinant intimin protein. (A) PCR product on 1% agarose gel. M, DNA size marker; lane 1, eae gene in pET28a plasmid. (B) Expression of the recombinant intimin on sodium dodecyl sulfate polyacrylamide gel electrophoresis gel 12%. Lane 1, before induction; lane 2, pellet of lysed bacteria; lane 3, supernatant from the lysate bacteria; lane 4, protein size marker; lane 5, samples of 2 hours inducted by IPTG (clone 1); lane 6, sample 16 hours inducted by IPTG (clone 1); lane 7, samples of 2 hours inducted by IPTG clone number 2; lane 8, sample 16 hours inducted by IPTG (clone 2); lane 9, samples of 2 hours inducted by IPTG (clone 3). (C) Intimin protein purification by gradient pH method. Lane 1, MES solution; lane 2, flow through; lane 3, washing solution (pH 5.7); lane 4, elution solution 1 (pH 4.5); lane 5, elution solution 2 (pH 4.5); lane 6, elution solution 3 (pH 4.5); lane 7, elution solution 4, (pH 4.5); lane 8, elution solution 5 (pH 4.5); lane M, protein size marker; lane 9, lysate bacteria sample. (D) Western Blotting of the recombinant intimin protein. Lane M, Protein size marker, Lane 1, non inducted bacteria sample, Lane 2, Recombinant intimin protein sample.
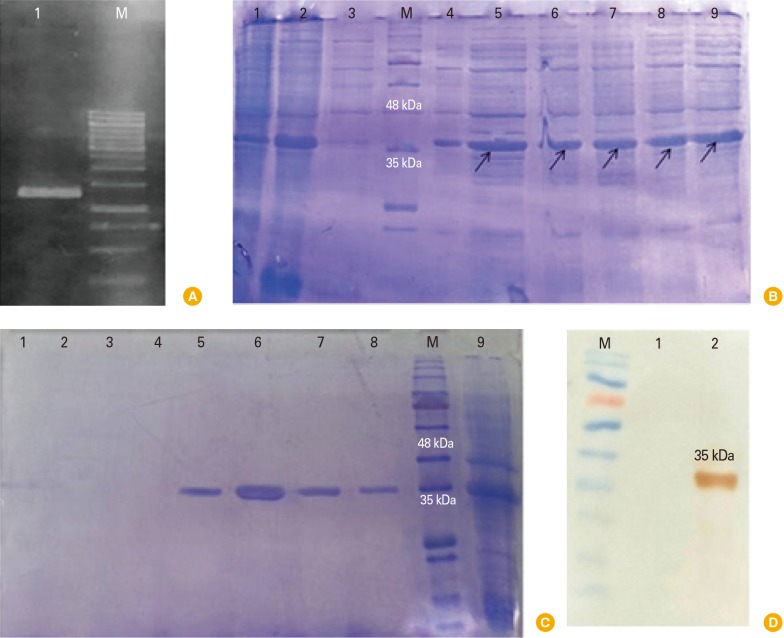
Fig. 3
Specific serum and fecal antibody titer against nanovaccine containig recombinant intimin and evaluation its shedding after gavaged with 109 of Escherichia coli O157:H7. (A) Serum IgG titers the groups. (B) Serum IgA titers among the groups. (C) Fecal IgA titers among the groups. (D) Fecal shedding results among the groups.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download