Introduction
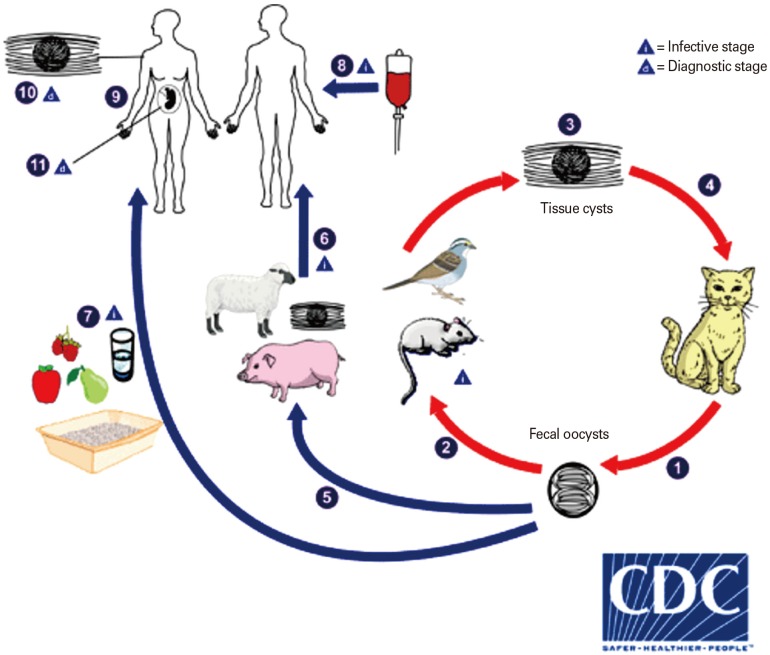 | Fig. 1The biologic stages and the main transmission routes of Toxoplasma gondii. The only known definitive hosts for T. gondii are members of family Felidae (domestic cats and their relatives). Unsporulated oocysts are shed in the cat's feces (1). Although oocysts are usually only shed for 1–2 weeks, large numbers may be shed. Oocysts take 1–5 days to sporulate in the environment and become infective. Intermediate hosts in nature (including birds and rodents) become infected after ingesting soil, water, or plant material contaminated with oocysts (2). Oocysts transform into tachyzoites shortly after ingestion. These tachyzoites localize in neural and muscle tissue and develop into tissue cyst bradyzoites (3). Cats become infected after consuming intermediate hosts harboring tissue cysts (4). Cats may also become infected directly by ingestion of sporulated oocysts. Animals bred for human consumption and wild game may also become infected with tissue cysts after ingestion of sporulated oocysts in the environment (5). Humans can become infected by any of several routes: eating undercooked meat of animals harboring tissue cysts (6); consuming food or water contaminated with cat feces or by contaminated environmental samples (such as fecal-contaminated soil or changing the litter box of a pet cat) (7); blood transfusion or organ transplantation (8); transplacentally from mother to fetus (9). Diagnosis is usually achieved by serology, although tissue cysts may be observed in stained biopsy specimens (10). Diagnosis of congenital infections can be achieved by detecting T. gondii DNA in amniotic fluid using molecular methods such as polymerase chain reaction (11) Adapted from Centers for Disease Control and Prevention [20]. |
Vaccine Candidate: Current State and Future
-
‒ Vaccination to prevent acute parasitemia and protect against congenital toxoplasmosis
‒ Prevent or reduce tissue cysts in food animals to interrupt the transmission route to humans‒ Prevention or reduction of oocyst shedding in cats to confine environmental contamination as well as minimize the risk of toxoplasmosis for all intermediate hosts
Calcium-Dependent Protein Kinase
Table 1
The main features and functions of some CDPKs
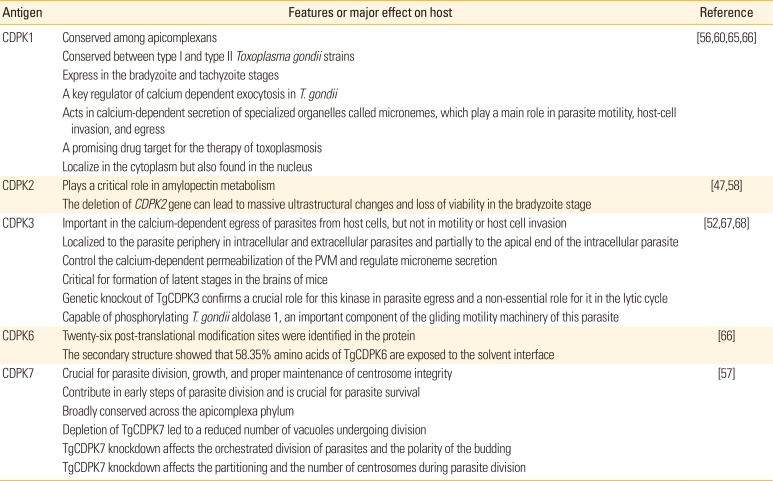
| Antigen | Features or major effect on host | Reference |
|---|---|---|
| CDPK1 | Conserved among apicomplexans | [56,60,65,66] |
| Conserved between type I and type II Toxoplasma gondii strains | ||
| Express in the bradyzoite and tachyzoite stages | ||
| A key regulator of calcium dependent exocytosis in T. gondii | ||
| Acts in calcium-dependent secretion of specialized organelles called micronemes, which play a main role in parasite motility, host-cell invasion, and egress | ||
| A promising drug target for the therapy of toxoplasmosis | ||
| Localize in the cytoplasm but also found in the nucleus | ||
| CDPK2 | Plays a critical role in amylopectin metabolism | [47,58] |
| The deletion of CDPK2 gene can lead to massive ultrastructural changes and loss of viability in the bradyzoite stage | ||
| CDPK3 | Important in the calcium-dependent egress of parasites from host cells, but not in motility or host cell invasion | [52,67,68] |
| Localized to the parasite periphery in intracellular and extracellular parasites and partially to the apical end of the intracellular parasite | ||
| Control the calcium-dependent permeabilization of the PVM and regulate microneme secretion | ||
| Critical for formation of latent stages in the brains of mice | ||
| Genetic knockout of TgCDPK3 confirms a crucial role for this kinase in parasite egress and a non-essential role for it in the lytic cycle | ||
| Capable of phosphorylating T. gondii aldolase 1, an important component of the gliding motility machinery of this parasite | ||
| CDPK6 | Twenty-six post-translational modification sites were identified in the protein | [66] |
| The secondary structure showed that 58.35% amino acids of TgCDPK6 are exposed to the solvent interface | ||
| CDPK7 | Crucial for parasite division, growth, and proper maintenance of centrosome integrity | [57] |
| Contribute in early steps of parasite division and is crucial for parasite survival | ||
| Broadly conserved across the apicomplexa phylum | ||
| Depletion of TgCDPK7 led to a reduced number of vacuoles undergoing division | ||
| TgCDPK7 knockdown affects the orchestrated division of parasites and the polarity of the budding | ||
| TgCDPK7 knockdown affects the partitioning and the number of centrosomes during parasite division |
CDPK DNA Vaccine
-
- Design (more rapid design as well as can be rapidly isolated and cloned)
- Versatility (ease in adapting or improving plasmid sequence, capability to deliver multi-antigen vaccines into a host only with a single dose, ease in formulation with different adjuvants)- Production (cost effective, ease of production, capable of large-scale production, appropriate protein folding for correct epitope expression)- Transport (stability at room temperature and no need to cold chain)- Safety (cannot revert to the pathogenic form, safer than live or attenuated vaccines)- Immune responses (boost the expression of an encoded vaccine antigen lwithin host cells, able to induce a longlasting immunity, elicits efficient and specific humoral and cellular immune responses, provide immune priming but poor immune boosting)
-
- Augment the immunogenicity of DNA vaccines
- Boost DNA delivery- Increased the magnitude/duration of plasmid DNA expression- Recruit the immune cells to the site of inoculation, thereby increases the immunostimulatory features of plasmid- Help the uptake of DNA into host cells or by professional antigen-presenting cells (APCs)- Protect plasmid against degradation by DNAses
Table 2
Baseline characteristics of included studies based on immunization experiments with DNA or protein vaccines against Toxoplasma gondii in mouse models
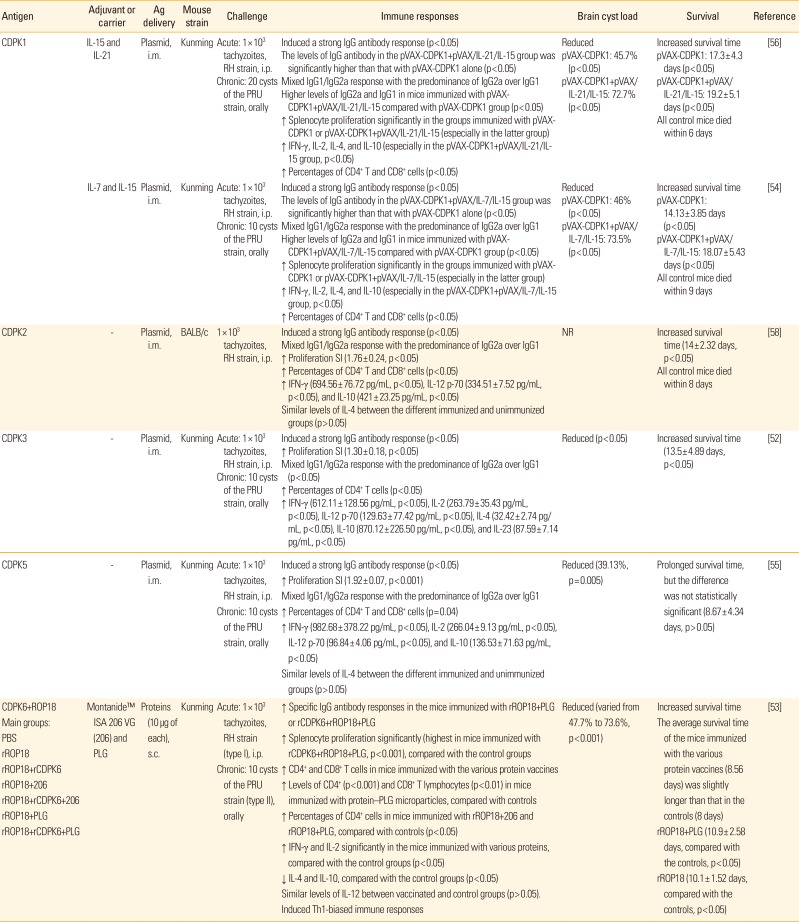
| Antigen | Adjuvant or carrier | Ag delivery | Mouse strain | Challenge | Immune responses | Brain cyst load | Survival | Reference |
|---|---|---|---|---|---|---|---|---|
| CDPK1 | IL-15 and IL-21 | Plasmid, i.m. | Kunming | Acute: 1×103 tachyzoites, RH strain, i.p. | Induced a strong IgG antibody response (p<0.05) | Reduced | Increased survival time | [56] |
| Chronic: 20 cysts of the PRU strain, orally | The levels of IgG antibody in the pVAX-CDPK1+pVAX/IL-21/IL-15 group was significantly higher than that with pVAX-CDPK1 alone (p<0.05) | pVAX-CDPK1: 45.7% (p<0.05) | pVAX-CDPK1: 17.3± 4.3 days (p<0.05) | |||||
| Mixed IgG1/IgG2a response with the predominance of IgG2a over IgG1 | pVAX-CDPK1+pVAX/IL-21/IL-15: 72.7% (p<0.05) | pVAX-CDPK1+pVAX/IL-21/IL-15: 19.2± 5.1 days (p<0.05) | ||||||
| Higher levels of IgG2a and IgG1 in mice immunized with pVAX-CDPK1+pVAX/IL-21/IL-15 compared with pVAX-CDPK1 group (p<0.05) | All control mice died within 6 days | |||||||
| ↑ Splenocyte proliferation significantly in the groups immunized with pVAX-CDPK1 or pVAX-CDPK1+pVAX/IL-21/IL-15 (especially in the latter group) | ||||||||
| ↑ IFN-γ, IL-2, IL-4, and IL-10 (especially in the pVAX-CDPK1+pVAX/IL-21/IL-15 group, p<0.05) | ||||||||
| ↑ Percentages of CD4+ T and CD8+ cells (p<0.05) | ||||||||
| IL-7 and IL-15 | Plasmid, i.m. | Kunming | Acute: 1×103 tachyzoites, RH strain, i.p. | Induced a strong IgG antibody response (p<0.05) | Reduced | Increased survival time | [54] | |
| Chronic: 10 cysts of the PRU strain, orally | The levels of IgG antibody in the pVAX-CDPK1+pVAX/IL-7/IL-15 group was significantly higher than that with pVAX-CDPK1 alone (p<0.05) | pVAX-CDPK1: 46% (p<0.05) | pVAX-CDPK1: 14.13±3.85 days (p<0.05) | |||||
| Mixed IgG1/IgG2a response with the predominance of IgG2a over IgG1 | pVAX-CDPK1+pVAX/IL-7/IL-15: 73.5% (p<0.05) | pVAX-CDPK1+pVAX/IL-7/IL-15: 18.07± 5.43 days (p<0.05) | ||||||
| Higher levels of IgG2a and IgG1 in mice immunized with pVAX-CDPK1+pVAX/IL-7/IL-15 compared with pVAX-CDPK1 group (p<0.05) | All control mice died within 9 days | |||||||
| ↑ Splenocyte proliferation significantly in the groups immunized with pVAX-CDPK1 or pVAX-CDPK1+pVAX/IL-7/IL-15 (especially in the latter group) | ||||||||
| ↑ IFN-γ, IL-2, IL-4, and IL-10 (especially in the pVAX-CDPK1+pVAX/IL-7/IL-15 group, p<0.05) | ||||||||
| ↑ Percentages of CD4+ T and CD8+ cells (p<0.05) | ||||||||
| CDPK2 | - | Plasmid, i.m. | BALB/c | 1×103 tachyzoites, RH strain, i.p. | Induced a strong IgG antibody response (p<0.05) | NR | Increased survival time (14±2.32 days, p<0.05) | [58] |
| Mixed IgG1/IgG2a response with the predominance of IgG2a over IgG1 | All control mice died within 8 days | |||||||
| ↑ Proliferation SI (1.76±0.24, p<0.05) | ||||||||
| ↑ Percentages of CD4+ T and CD8+ cells (p<0.05) | ||||||||
| ↑ IFN-γ (694.56±76.72 pg/mL, p<0.05), IL-12 p-70 (334.51±7.52 pg/mL, p<0.05), and IL-10 (421±23.25 pg/mL, p<0.05) | ||||||||
| Similar levels of IL-4 between the different immunized and unimmunized groups (p>0.05) | ||||||||
| CDPK3 | - | Plasmid, i.m. | Kunming | Acute: 1×103 tachyzoites, RH strain, i.p. | Induced a strong IgG antibody response (p<0.05) | Reduced (p<0.05) | Increased survival time (13.5±4.89 days, p<0.05) | [52] |
| Chronic: 10 cysts of the PRU strain, orally | ↑ Proliferation SI (1.30±0.18, p<0.05) | |||||||
| Mixed IgG1/IgG2a response with the predominance of IgG2a over IgG1 (p<0.05) | ||||||||
| ↑ Percentages of CD4+ T cells (p<0.05) | ||||||||
| ↑ IFN-γ (612.11±128.56 pg/mL, p<0.05), IL-2 (263.79±35.43 pg/mL, p<0.05), IL-12 p-70 (129.63±77.42 pg/mL, p<0.05), IL-4 (32.42±2.74 pg/mL, p<0.05), IL-10 (870.12± 226.50 pg/mL, p<0.05), and IL-23 (87.59±7.14 pg/mL, p<0.05) | ||||||||
| CDPK5 | - | Plasmid, i.m. | Kunming | Acute: 1×103 tachyzoites, RH strain, i.p. | Induced a strong IgG antibody response (p<0.05) | Reduced (39.13%, p=0.005) | Prolonged survival time, but the difference was not statistically significant (8.67±4.34 days, p>0.05) | [55] |
| Chronic: 10 cysts of the PRU strain, orally | ↑ Proliferation SI (1.92±0.07, p<0.001) | |||||||
| Mixed IgG1/IgG2a response with the predominance of IgG2a over IgG1 | ||||||||
| ↑ Percentages of CD4+ T and CD8+ cells (p=0.04) | ||||||||
| ↑ IFN-γ (982.68±378.22 pg/mL, p<0.05), IL-2 (266.04±9.13 pg/mL, p<0.05), IL-12 p-70 (96.84±4.06 pg/mL, p<0.05), and IL-10 (136.53±71.63 pg/mL, p<0.05) | ||||||||
| Similar levels of IL-4 between the different immunized and unimmunized groups (p>0.05) | ||||||||
| CDPK6+ROP18 | Montanide™ ISA 206 VG (206) and PLG | Proteins (10 µg of each), s.c. | Kunming | Acute: 1×103 tachyzoites, RH strain (type I), i.p. | ↑ Specific IgG antibody responses in the mice immunized with rROP18+PLG or rCDPK6+rROP18+PLG | Reduced (varied from 47.7% to 73.6%, p<0.001) | Increased survival time | [53] |
|
Main groups: PBS rROP18 rROP18+rCDPK6 rROP18+206 rROP18+rCDPK6+206 rROP18+PLG rROP18+rCDPK6+PLG |
Chronic: 10 cysts of the PRU strain (type II), orally | ↑ Splenocyte proliferation significantly (highest in mice immunized with rCDPK6+rROP18+PLG, p<0.001), compared with the control groups | The average survival time of the mice immunized with the various protein vaccines (8.56 days) was slightly longer than that in the controls (8 days) | |||||
| ↑ CD4+ and CD8+ T cells in mice immunized with the various protein vaccines | rROP18+PLG (10.9±2.58 days, compared with the controls, p<0.05) | |||||||
| ↑ Levels of CD4+ (p<0.001) and CD8+ T lymphocytes (p<0.01) in mice immunized with protein–PLG microparticles, compared with controls | rROP18 (10.1±1.52 days, compared with the controls, p<0.05) | |||||||
| ↑ Percentages of CD4+ cells in mice immunized with rROP18+206 and rROP18+PLG, compared with controls (p<0.05) | ||||||||
| ↑ IFN-γ and IL-2 significantly in the mice immunized with various proteins, compared with the control groups (p<0.05) | ||||||||
| ↓ IL-4 and IL-10, compared with the control groups (p<0.05) | ||||||||
| Similar levels of IL-12 between vaccinated and control groups (p>0.05). | ||||||||
| Induced Th1-biased immune responses |




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download