This article has been
cited by other articles in ScienceCentral.
Abstract
Purpose
There is a need to broaden protective coverage of M protein–based vaccines against group A streptococci (GAS) because coverage of the current 30-valent M protein vaccine does not extend to all emm types. An additional GAS antigen and virulence factor that could potentially extend vaccine coverage is M-related protein (Mrp). Previous work indicated that there are three structurally related families of Mrp (MrpI, MrpII, and MrpIII) and peptides of all three elicited bactericidal antibodies against multiple emm types. The purpose of this study was to determine if a recombinant form containing Mrp from the three families would evoke bactericidal antiserum and to determine if this antiserum could enhance the effectiveness of antisera to the 30-valent M protein vaccine.
Materials and Methods
A trivalent recombinant Mrp (trMrp) protein containing N-terminal fragments from the three families (trMrp) was constructed, purified and used to immunize rabbits. Anti-trMrp sera contained high titers of antibodies against the trMrp immunogen and recombinant forms representing MrpI, MrpII, and MrpIII.
Results
The antisera opsonized emm types of GAS representing each Mrp family and also opsonized emm types not covered by the 30-valent M protein–based vaccine. Importantly, a combination of trMrp and 30-valent M protein antiserum resulted in higher levels of opsonization of GAS than either antiserum alone.
Conclusion
These findings suggest that trMrp may be an effective addition to future constructs of GAS vaccines.
Keywords: Streptococcus pyogenes, Vaccines, Virulence factors
Introduction
Group A streptococcus (GAS) is a significant cause of morbidity and mortality worldwide [
1]. This pathogen is responsible for illnesses ranging from acute pharyngitis and skin infections to more serious invasive infections and autoimmune post-infectious complications that include glomerulonephritis, rheumatic fever, and rheumatic heart disease. Current efforts to develop safe and effective vaccines to prevent GAS infections have primarily focused on the surface M protein (also termed Emm and its gene as
emm) as the principal vaccine antigen [
2]. The major obstacle in M protein–based vaccine development has been the number of different
emm types of GAS (>200), all of which have different N-terminal protective epitopes that elicit
emm type-specific immunity [
3]. This has necessitated the development of highly complex recombinant multivalent vaccines containing up to 30 type-specific M peptides linked in tandem [
3]. Recent findings have indicated that coverage by the 30-valent M protein vaccine is more extensive than predicted by its components [
4]. However, despite the complexity, these vaccines do not provide potential coverage against all
emm types of GAS, in particular many of those prevalent in developing countries where populations are at greatest risk for acute rheumatic fever and rheumatic heart disease [
5]. This has prompted the search for additional antigens that may contain protective epitopes that are shared among many or all serotypes of GAS. The addition of these antigens could broaden the potential efficacy of M protein–based vaccines.
One such antigen, M-related protein (Mrp), a virulence factor of GAS [
6789], has recently been considered as a potential vaccine component [
10]. Mrp is member of the family of M proteins, but unlike M proteins which have hypervariable N-termini, the N-termini of Mrp are semi-conserved. All of the Mrps that have been sequenced to date fall into three structurally related groups, MrpI, MrpII, and MrpIII [
10]. Mrps are expressed by 83% of clinical isolates of GAS and evoke protective antibodies in rabbits and humans [
610]. Antisera against individual recombinant peptides containing N-terminal sequences from each of the three structural groups were found to opsonize and promote phagocytic killing of GAS [
10]. These results suggested that Mrp may be used in conjunction with M proteins to broaden overall vaccine coverage of GAS infections in developing countries of the world. The present study was designed to evaluate the potential efficacy of a new trivalent recombinant Mrp (trMrp) vaccine alone and in combination with the current 30-valent vaccine construct.
Materials and Methods
Construction, expression, and purification of recombinant proteins
The construction of recombinant proteins encompassing the N-terminal domains of each of the Mrp families was previously described [
10]. The trMrp was constructed by synthesizing in tandem the DNA encoding the mature N-terminal fragments from Mrp49 (83 amino acids), Mrp4 (83 amino acids), and Mrp2 (93 amino acids) representing the groups MrpIII, MrpII, and MrpI respectively, followed by DNA encoding a polyhistidine metal binding site (Genescript, Piscataway, NJ, USA) (
Fig. 1). The synthetic gene was also designed to contain an upstream T7 promoter for protein expression. The product was ligated into pUC57, introduced into
Escherichia coli C3013, and expressed as a histidine fusion product. The recombinant proteins were purified by nickel-metal affinity chromatography and purity assessed by polyacrylamide gel electrophoresis.
Vaccine formulation and immunization of rabbits
Three New Zealand white rabbits were immunized intramuscularly with 150 µg of trMrp adsorbed to an equal amount of 2% aluminum hydroxide gel (wt/wt) at time 0 week, 4 weeks, and 8 weeks. A booster injection was given at 12 weeks and blood was obtained 2 weeks after the final injection by ear venipuncture. Rabbit sera were collected after clotting of blood and centrifugation.
Enzyme linked immunosorbent assay
Rabbit antisera against trMrp were assayed by enzyme linked immunosorbent assay (ELISA) using previously described methods [
11] with recombinant proteins representing each Mrp family and trMrp as solid-phase antigens. Microtiter wells were coated with recombinant proteins (5 µg/mL in 0.01 M sodium bicarbonate, pH 9.5 for 1 hour at 37℃). Control wells were coated with bovine serum albumin (BSA). After being coated, all wells were blocked with BSA (1 mg/mL in phosphate buffered saline) for one hour at 37℃. Serial 1:2 dilutions of rabbit sera were added to the wells and incubated for 1 hour at 37℃. The wells were washed, and a 1:2,000 dilution of peroxidase-labeled goat anti-rabbit immunoglobulins was added. After 1 hour at 37℃, the wells were washed and the substrate ABTS (2,2′-azino-di(3-ethyl-benzthiazoline-6-sulfate)) was added. After color development, the absorbance at 405 nm was measured. The average value for wells coated with BSA served as a blank and was subtracted from all other values. All samples were tested in duplicate. The titers were determined as the reciprocal of the last dilution that provided a reading greater than 0.1 at 405 nm.
Bactericidal assays
Bactericidal assays were performed as previously described [
10]. Briefly, 0.01 mL of Todd-Hewitt broth containing log-phase bacteria was added to 0.05 mL of test serum or normal rabbit serum (NRS). Then 0.140 mL of lightly heparinized, non-immune human blood was added and the complete mixture was rotated for 2 hours at 37℃. Bacterial cell numbers in the samples were determined by plating serial dilutions on blood agar plates and counting the number of colony forming units (CFU) after incubating the plates overnight at 37℃. The results were expressed as percent killing, calculated using the formula: {[1-(total CFU with test serum/total CFU with NRS)] ×100}. Only donors were used whose blood resulted in growth of the test strain to at least four generations in the presence of NRS, thus avoiding donors with opsonic antibodies in their blood.
Some bactericidal assays were performed using a mixture of trMrp vaccine and 30-valent vaccine. In these studies, equal parts trMrp vaccine antiserum and 30-valent M vaccine antiserum were used to maintain a fixed volume of 0.05 mL immune rabbit serum in the assay. Controls for these assays included 30-valent and trMrp vaccine antiserum diluted 1:2 with NRS.
Ethics statement
The animal studies were performed after receiving approval of the Institutional Animal Care and Use Committee (IACUC No. 15-125.0) and the experiments involving human blood were approved by the Institutional Review Board (IRB No. 90-04041-xp) at the University of Tennessee Health Science Center, Memphis, TN, USA.
Results
Binding specificity of Mrp antibodies
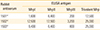
Immunization of the rabbits with trMrp evoked antibodies against each component of the vaccine. Individual recombinant Mrp peptides representing the structures in each of the three Mrp groups (MrpI, MrpII, and MrpIII) and trMrp were used as solid-phase antigens in an ELISA in order to determine if antibodies were developed against each Mrp family in the trivalent vaccine. The immune sera reacted in high titer with the trivalent vaccine recombinant protein and with recombinant proteins from each member of the Mrp families as well (
Table 1). There was some degree of variability in the rabbits' immune responses to the trMrp vaccine. Rabbits 1502 and 1503 developed high titers of antibodies against each of the recombinant proteins. Although rabbit 1501 also developed antibodies to each of the recombinant proteins, the titers were lower.
trMrp vaccine evokes opsonic antibodies against GAS
Bactericidal assays were performed using the trMrp vaccine immune sera against several GAS types expressing Mrp proteins from Mrp groups I, II, and III (
Fig. 2). M5, a serotype that does not express Mrp, was used as a negative control and was not opsonized by the trMrp antisera. trMrp vaccine sera promoted bactericidal activity against multiple
emm types of GAS that expressed Mrp's from all three structurally related families (
Fig. 2). It is important to note that among these serotypes of GAS are several M types (M86, M88, and M108) that are not represented in the current 30-valent vaccine. Linking the N-terminal peptides in tandem did not change the opsonic activity of the immune sera compared to results previously reported for antisera raised against the individual Mrp peptides [
10].
The 30-valent vaccine antiserum and the trMrp vaccine antiserum were mixed together and used in bactericidal assays to determine if combinations of Mrp and M antibodies enhanced opsonization and phagocyte-mediated killing of GAS (
Fig. 3). Suboptimal concentrations of antibodies were used (diluted 1:2 with NRS) in order to demonstrate increased killing when the two sera were combined, while maintaining the total volume of serum in the assay. In all cases, combinations of 30-valent and trMrp immune sera produced a higher level of killing than either serum alone (
Fig. 3).
Discussion
The acquisition of M protein antibodies that promote bactericidal activity following natural infection has been well described and serves as the basis for current M protein–based vaccines [
12]. Despite demonstrated efficacy, there are several issues with M protein–based vaccines that must be addressed in next generation vaccine development. First, the current 30-valent M protein–based vaccine will not provide optimal coverage against all
emm types of GAS, especially those circulating in developing countries where the epidemiology of GAS varies from that of developed countries [
5]. Second, immunogenicity studies of a previous 26-valent M protein–based vaccine revealed that the immune response, as measured by antibody titer, was not uniform among the 26 individual M peptides contained within the vaccine, nor was it uniform among vaccinated individuals [
13]. These challenges have prompted the search for additional surface antigens containing protective epitopes that are shared among many or all
emm types of GAS. Mrp is one such antigen, which is expressed by a majority of clinical GAS isolates. It has been demonstrated that Mrp antibodies are acquired following natural infection in the same age-related manner as M protein antibodies [
10]. Additionally, antibodies raised in rabbits against recombinant and synthetic N-terminal Mrp peptides were bactericidal [
1011].
The aim of this study was to evaluate the immunogenicity of a new recombinant trMrp vaccine and to test the bactericidal activity of immune sera alone and in combination with antisera against the current 30-valent vaccine. ELISA studies confirmed high titer antibodies to each of the individual Mrp proteins as well as the recombinant trMrp vaccine protein. The trMrp vaccine antisera were bactericidal against various GAS serotypes including those expressing MrpI, MrpII, and MrpIII. Combinations of 30-valent M protein–based vaccine antiserum and trMrp vaccine antiserum were used in bactericidal assays to evaluate the potential efficacy of a more complex vaccine. The findings indicate that combinations of antisera are more functional than either antiserum alone. Taken together, these studies suggest that the trMrp peptide would be a beneficial addition to next generation M protein–based vaccines. Additionally, Mrp antibodies could potentially enhance protection against GAS infection in individuals that mount a suboptimal immune responses to select M peptides in the current 30-valent vaccine. Future studies will include the use of combination M and Mrp vaccines in animal models of GAS infection to confirm enhanced protective efficacy with combination vaccines. Although M protein–based vaccines have been the backbone of GAS vaccine development for decades, next generation vaccines will likely be designed to include additional antigens, such as Mrp, to broaden coverage and enhance global efficacy.
Figures and Tables
 | Fig. 1Schematic of the trivalent recombinant M-related protein (Mrp) vaccine construct. Mrps comprise three structurally related families: MrpI (represented by Mrp2), MrpII (represented by Mrp4), and MrpIII (represented by Mrp49).
|
 | Fig. 2Bactericidal activity of trivalent M-related protein (Mrp) vaccine rabbit antisera against multiple group A streptococci (GAS) emm types expressing Mrp from the three Mrp families, Mrp I-III. M5 GAS does not express Mrp and served as a negative control. Bars represent the mean±standard error of mean of four independent experiments.
|
 | Fig. 3Combinations of 30-valent M protein and trivalent M-related protein (Mrp) (trMrp) antisera promote enhanced bactericidal killing against multiple Mrp-positive group A streptococci (GAS) emm types. Antisera to trMrp (blue column) or to 30-valent M protein vaccine (orange column) were used alone or in combination with each other (red column). Antisera were mixed with an equal volume of normal rabbit serum when used singly and equal volumes of each when combined. Bars represent the mean±standard error of mean of four independent experiments. The asterisk above the bars indicates that the combination of anti-trMrp and anti-30-valent sera was significantly different in killing (p<0.05) than either antisera alone.
|
Table 1
Trivalent Mrp vaccine rabbit antiserum titers against individual Mrp peptides and the immunizing trivalent Mrp protein
|
Rabbit antiserum |
ELISA antigen |
|
MrpI |
MrpII |
MrpIII |
Trivalent Mrp |
|
1501a)
|
1,600 |
6,400 |
200 |
12,500 |
|
1502a)
|
12,500 |
12,500 |
3,200 |
25,000 |
|
1503a)
|
6,400 |
6,400 |
800 |
25,000 |








 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download