This article has been
cited by other articles in ScienceCentral.
Abstract
Vaccination is the most efficient method for infectious disease prevention. Parenteral injections such as intramuscular, intradermal, and subcutaneous injections have several advantages in vaccine delivery, but there are many drawbacks. Thus, the development of a new vaccine delivery system has long been required. Recently, microneedles have been attracting attention as new vaccination tools. Microneedle is a highly effective transdermal vaccine delivery method due to its mechanism of action, painlessness, and ease of use. Here, we summarized the characteristics of microneedles and the possibilities as a new vaccine delivery route.
Keywords: Microneedles, Vaccines, Transdermal drug delivery system, Needle-free vaccination
Vaccination is the immunological preparation of the adaptive immune system for a specific disease. It reduces the incidence of infectious diseases in childhood and prolongs the life span [
1]. Therefore, vaccines are commonly used in our community. Vaccines can be classified into several types depending on the formulation. Live-attenuated vaccines contain live viruses which cannot cause disease in healthy individuals. Inactivated or killed vaccines use inactivated forms of microbial pathogens. Toxoid vaccines contain weakened toxins derived from pathogenic bacterial products. Subunit vaccines contain certain parts of the virus or bacteria's antigens. The conjugated vaccine contains a polysaccharide composed of bacterial outer membrane as an antigen [
2]. Peptide vaccines consist of synthetic peptides which can cause an adoptive immune response. DNA vaccines comprise a specific genetic part of the pathogen.
For the vaccine development, several characteristics such as safety, stability, cost-effectiveness, ease of dispense, and ability to trigger an effective immune response must be considered. To ensure safe and effective delivery of the vaccine, the route of vaccination has been recognized as an important point [
3]. Common methods of administration of vaccines include intramuscular (IM), intradermal (ID), subcutaneous (SC), and oral administration [
4]. IM injections are commonly used for vaccine delivery because more blood vessels are distributed around the muscles than the skin. ID and SC injections are also often used for delivery of some vaccines, and small number of vaccines is administered via the oral route [
4].
Although parenteral injection routes are widely used, needles and syringes create problems such as needle-phobia or accidents caused by used needles [
5]. Based on the limitation of parenteral injections, various needle-free methods have been proposed for many years. One of them is the mucosal surface vaccination. It focuses on the immune response caused by dendritic cells and lymphocytes circulating in the mucosal space [
6]. Administration via the nasal surface (intranasal) or sublingual route is a typical example of a mucosal route. Additionally, pulmonary, vaginal and rectal routes are considered as other delivery routes; however, they have some disadvantages. The pulmonary route requires special devices and the vaginal or rectal route can cause discomfort to the patient [
5]. Above all, more research is needed on the safety and efficacy of needle-free vaccination compared to the conventional vaccination [
47]. These drawbacks require novel strategies for delivering the vaccine through the skin.
Skin is one of the best targets for drug delivery because it is the largest organ that surrounds the body that takes up about one-third of the blood circulation in our body and is responsible for the absorption of chemical- and bio-agents [
8]. Transdermal drug delivery system (TDDS) is a delivery method that attaches to the skin and delivers the drug directly through the skin. It can compensate the disadvantages of oral and SC administration, which are currently used as drug delivery methods. TDDS can be categorized as three undergoing generations of development.
The first-generation TDDS is continuously being used in the clinical settings for delivery of small, lipophilic, low-dose drugs using the direct route through the skin. Due to advances in patch technology, the number of first-generation transdermal patches on the market has surged recently. However, this surge will gradually diminish as drugs with properties suitable for such systems become depleted [
9]. The second-generation TDDS was developed to enhance the skin permeability of transdermal drugs. It uses biochemical enhancers, non-cavitational ultrasound, and iontophoresis. However, the method developed in this generation suffers from a balance between achieving increased delivery across the stratum corneum and protecting deeper tissues from damage. Thus, this second-generation TDDS did not significantly affect the delivery of macromolecules [
910]. The third-generation TDDS can have a significant impact on drug delivery because they target the effects on the stratum corneum. This targeting allows stronger disruption of the stratum corneum barrier while protecting deeper tissues, enabling more effective transdermal delivery. Microneedles, thermal ablation, microdermabrasion, electroporation, and cavitational ultrasound have been shown to deliver macromolecules including therapeutic proteins and vaccines [
9].
Recently, there have been many researches on microneedles that combine a conventional injection system with a patch system. The microneedles physically puncture the stratum corneum, the outermost layer of the skin, and deliver the drug through the holes. It can deliver drugs faster and more efficiently than conventional transdermal delivery systems. Microneedles consist of quadrangular pyramids or cones of tens or hundreds of micrometers long. It is a highly effective transdermal drug delivery method because of its sustained drug release, ease of use, and painlessness. In addition, microneedles can substantially reduce logistics costs and waste and are easy to store. These advantages show that microneedles are suitable for vaccination routes [
111213].
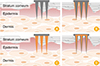
Microneedles can generally be classified into four types according to drug delivery methods: solid, coated, dissolving, and hollow microneedles (
Fig. 1) [
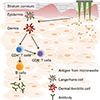
13]. Each type of microneedle has pros and cons. A solid microneedle punctures the surface of the skin and applies the drug to the skin layer, allowing the drug to slowly diffuse through the holes. It has the advantage of preventing pathogenic infection but the drug delivery effect is low. Coated microneedle is typically coated with a water-soluble drug. The microneedle is attached to the skin, the drug is quickly delivered to the skin, and then the microneedle is removed. It has an advantage of delivering a very small fixed amount of drug, but the remaining microneedle tips are dangerous because they can infect other people. Unlike other types of microneedle, the dissolving microneedles are made of water-soluble materials. When the microneedle is pushed into the skin, the microneedle melts in the skin, releasing the drug in the microneedle. Dissolving microneedles improves the disadvantages of the coated microneedles by transferring large doses of drug, but it is difficult to deliver a fixed amount of drug. Finally, the hollow microneedles are similar to a conventional syringe of short length in shape, allowing liquid medication to be injected directly into the skin layer. Since antigen-presenting cells are largely distributed on the skin, the benefits of vaccines are even greater when using microneedles (
Fig. 2) [
12]. Recently, dissolving and hollow microneedles have been developed for vaccine delivery.
Although there are clinical trials for hepatitis B, varicella, and poliomyelitis, most studies on vaccines using microneedle are about the influenza infection [
111415]. Various pre-clinical/clinical studies on vaccination using microneedle against influenza, along with the advantages mentioned above, provide the following features: vaccines using microneedle have relatively broader and higher immunogenicity, immunostability, strong cellular immune response, rapid viral clearance, and dose reduction compared to IM- or SC-injections [
16].
Vaccines using microneedle formulations have been developed for a variety of infectious diseases, such as anthrax, chikungunya, diphtheria, hepatitis B, hepatitis C, herpes simplex, human papillomavirus infection, influenza, poliomyelitis, measles, rabies, rotavirus infection, plaque, tetanus, tuberculosis, and West Nile fever [
16]. In order to achieve successful commercialization of vaccines using microneedle, it is necessary to establish validation of TDDS. In addition, if low-cost, stable vaccines using microneedle are produced, many research infrastructures will eventually lead to the development of new vaccine formulations that can be used in clinical medicine.
Although the development period of the microneedle technique such as TDDS has not been so long, it has been remarkably developed as a drug administration method including vaccines and therapeutic agents. In particular, their ability to load and subsequent release into the skin barrier are special to improve pharmacokinetics as well as immunogenicity of the vaccine over conventional vaccine. Drug delivery systems based on microneedle techniques are potentially valuable in future vaccine delivery methods. With effective drug delivery, patient compliance, cost saving, and ease of storage, TDDS using microneedle technology will be a milestone in the development and dissemination of vaccines, and will contribute to human public health.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download