Introduction
Immunization is the most effective and cost-effective means of public health intervention. However, like other medications, vaccines are not perfect and may have associated risks. Therefore, national public health officials and the public health community must constantly monitor vaccine-related adverse events, assess risk, and inform the public of any harmful effects [
1]. As the incidence of vaccine-preventable diseases (VPDs) decreases, the concern surrounding adverse reactions after vaccination becomes more prominent. Public concerns about adverse reactions may cause delays to, or refusal of, vaccination. Even when a VPD has decreased in prevalence after the introduction of a vaccination, the subsequent reemergence of the infectious disease has occurred in many countries due to decreased immunization coverage [
234]. In order to sustain high coverage of vaccinations, public trust on vaccine safety must be retained, and there is a need for systematic institutional support in order to ensure a safe National Immunization Program (NIP).
In Korea, the media reported two cases of death following the Japanese encephalitis vaccination in 1994. Thereafter, vaccine safety became a social issue and, as concerns about the vaccine among the general public increased, so did the need for national management of vaccine safety. As a result, an amendment to the Prevention of Contagious Disease Act was passed in July 1994, and the Korea National Vaccine Injury Compensation Program (KVICP) was launched in January 1995 to allow the Korea Advisory Committee on Vaccine Injury Compensation (KACVIC) to determine whether an adverse event results from a vaccination [
5]. Subsequently, a surveillance system for adverse events following immunization (AEFI) was developed in 1999, and reporting requirements and obligations for adverse events were defined in 2000 [
6]. A more systematic approach to monitoring AEFI was established in Korea through these processes.
Academic research on reports of AEFI and national vaccine injury compensation in Korea has been intermittently published. A study regarding vaccine-related adverse events between 1995 and 2000 was published in 2001 [
6]. An introduction to the vaccine safety management system in Korea and a review of reports and claims filed regarding AEFIs from 1994 to 2010 was circulated in 2013 [
7]. However the NIP recently introduced new vaccines every year. Eight types of vaccines for children aged 12 years or less and one type of vaccine, 23-valent pneumococcal polysaccharide vaccine (PPV23), for the elderly aged 65 years and over have been introduced from 2011 to 2016. Consequently, the numbers of vaccine types and subjects filing compensation claims has increased. In addition, some new research with large-scale populations on vaccinations and AEFI has been released, and the standards for compensation of adverse events have changed with time. In this study, we reviewed and analyzed AEFI cases reported during the last 6 years (2011-2016) in the Republic of Korea.
Materials and Methods
Reported AEFIs and AEFI proportion by vaccine type
We reviewed all AEFIs registered in the Integrated Management System of Disease and Public Health from January 1, 2011 to December 31, 2016. Adverse events following vaccinations under the NIP, provisional vaccinations by local governments, and voluntarily administered vaccinations were registered to the AEFI surveillance system. For those cases reported as death or disability, additional data were collected, including the results of the rapid response team, information from the consultation meeting, and reevaluation of vaccine lot.
The number of vaccinations administered for each type of vaccine was collected through the National Immunization Registry Integration system of the Division of VPD Control and the NIP at Korea Centers for Disease Prevention and Control (KCDC). We calculated the AEFI reporting rate for each vaccine type by comparing the number of reported AEFI to the total number of vaccinations per year (number of AEFI cases per 100,000 administered doses). Even if two or more vaccine types contain the same antigen (e.g., diphtheria and tetanus toxoids and acellular pertussis [DTaP], tetanus and diphtheria toxoids [Td], and tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine [Tdap]; inactivated polio vaccine [IPV] and DTaP-IPV), we classified them as separate vaccines. In addition, if two or more vaccines were administered at the same time and an adverse event was reported, the adverse event was considered reported for each vaccine. For example, when bacillus Calmette–Guérin (BCG) and hepatitis B vaccines were administered together and the adverse reaction of fever was reported, this reaction was recorded as an adverse event for BCG vaccine and an adverse event for hepatitis B vaccine. Therefore, the total number of adverse events reported by vaccine type is higher than the total number of adverse events reported overall.
Eligibility and review process for compensation claims
Compensation claims for AEFIs are filed and reviewed according to the criteria presented in the Infectious Disease Control and Prevention Act [
8]. This act facilitates compensation for adverse events that occur after vaccinations included in the NIP, or after standard immunizations recommended by the government (e.g., the influenza vaccine recommended for pregnant women), although other vaccinations voluntarily undergone by individuals are not covered. Additionally, cases are only eligible for compensation when patient's copayment due to the adverse reaction amount to 300,000 Korean won (266 U.S. dollars) or more. In other words, the objective of the KVICP is to require the government to compensate for moderate or severe adverse events, but not for many minor events.
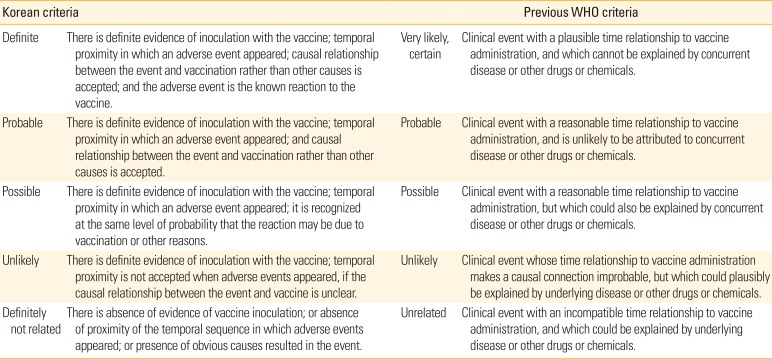
The KACVIC, which is composed of 15 expert members, reviews the causal association between adverse events and vaccine administration, and assesses whether each case meets the criteria for compensation. The KACVIC meets regularly once a quarter to evaluate each claim submitted to the Division of VPD Control and the NIP at each trial. Our classification of causality assessment is composed of five categories, which are based on the previous World Health Organization (WHO) causality assessment criteria [
9], but are modified according to our circumstances. The categories are as follows: definitely related, probably related (likely), possibly related, probably not related (unlikely), and definitely not related (
Table 1) [
8]. Recently, however, the WHO has simplified their causality assessment criteria to only three categories: consistent, inconsistent causal association to immunization, and indeterminate [
10]. When a temporal relationship is consistent but there is insufficient definitive evidence for adverse reaction, or evidences show conflicting trends of consistency and inconsistency, it is categorized to “indeterminate.” While our system uses five categories for causality assessment, there is no difference in the compensation amounts among cases in the “definitely related,” “probably related,” and “possibly related” categories; for all of these cases, the claimed amount is fully compensated. In contrast, cases categorized as “probably not related” and “definitely not related” did not constitute causality; thus, compensation for the amount claimed is totally rejected in these instances.
Analysis of claims filed for compensation
We reviewed the internal recordings from the Division of VPD Control and the NIP, including registered injury compensation applications, medical records, epidemiological investigations, and the results of injury compensation meetings. Claims can be filed once if there is a disagreement with the results of the review, and additional claims can be made if additional damages occur within 5 years of the disease diagnosis [
8]. In some cases, claims were changed from compensation for disease to compensation for disability when a patient received a diagnosis of disability following an illness. In such circumstances under which one patient claimed multiple compensations, we included each claim separately in the total and annual numbers of claims.
We compared age, sex, type of vaccine, time interval from vaccination to adverse event, type of adverse event, and underlying diseases between compensated and dismissed claims for compensation from 2011 to 2016. In order to define the major characteristics of the compensated and dismissed cases, we considered instances for which a single patient made multiple claims for compensation as one case, the most recent result of which we adopted as the final one. Four cases for which final deliberation was deferred were not included in the study. If more than two types of vaccine were administered simultaneously and an adverse event was reported, we regarded each vaccine as having its own adverse event.
Results
Adverse events reported to the AEFI surveillance system and claims filed for compensation from 2011 to 2016
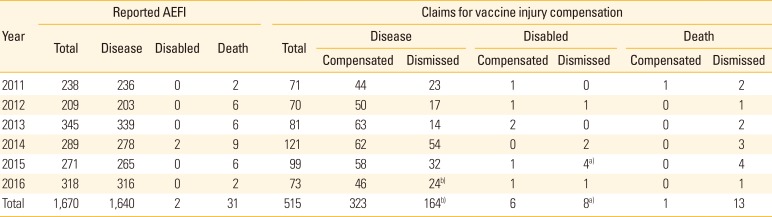
Table 2 shows the number of adverse events reported and claims filed between 2011 and 2016. An average of 278 cases (range, 209 to 345) was reported annually following vaccinations, including two cases of disability and 31 cases of death. The cases reported as disability involved immunization with DTaP-IPV and DTaP, followed by episodes diagnosed as epilepsy. In total, 31 deaths were reported, ranging from two to nine cases each year.
There was a range of 70 to 121 applications for compensation filed each year, totaling 515 applications over the 6-year period. Most of these were compensation claims for illness (487 cases, 94.5%), and two-thirds of them were awarded. In addition, 14 claims were filed for disability, six of which (43%) were compensated. Only one of 14 claims (7%) for death compensation was awarded. The case was 4-month-old baby who developed acute myocarditis and encephalopathy with multi-organ failure one day after DTaP and IPV inoculation.
Adverse events reported as death to the AEFI surveillance system
Among the 31 cases reported as death, 15 (48%) involved individuals aged 65 years or older, 12 (39%) involved infants under one year old, three (10%) involved adults, and one (3%) involved a child. The median ages were 77 years (range, 65 to 88 years) and 2 months (range, 0 to 10 months) for the elderly and infants, respectively. The male to female ratio differed by age group. While male subjects were predominant among the elderly (12 out of 15 individuals, 80%), only five out of 12 infant subjects (42%) were male. Whereas influenza and pneumococcal vaccines accounted for the majority of deaths in adults and the elderly (17 out of 18 cases), various vaccines accounted for infant deaths: DTaP-IPV (5), hepatitis B virus (HBV) (3), DTaP-IPV+HBV (1), and multi-vaccination including haemophilus influenzae type b vaccine (Hib) (3). The median time interval between vaccination and death was 1 day (range, 0 to 93 days).
Out of 31 death cases, autopsies were performed in 13 cases, 11 of which involved infants. Reevaluation of the vaccine lot was conducted in 12 cases, nine of which involved infants, and there was no causation due to vaccine lot. According to the results of the 11 infant autopsies performed, sudden infant death syndrome including asphyxia was suspected in seven cases, and unknown or insufficient cause of death was concluded in three cases. Among 12 infant deaths, only one individual was reported to have an underlying condition. In contrast, 10 out of 15 elderly subjects were reported to have an underlying disease. In all elderly death cases, causes of death other than vaccination, compounded by underlying disease and old age, were strongly suspected. Finally, rapid assessments for reported death cases were that 29 out of 31 death cases had low association with immunization, and two cases had inadequate information to evaluate causality, but, in which the vaccines were not suspected of causing deaths considering vaccine lot and other factors.
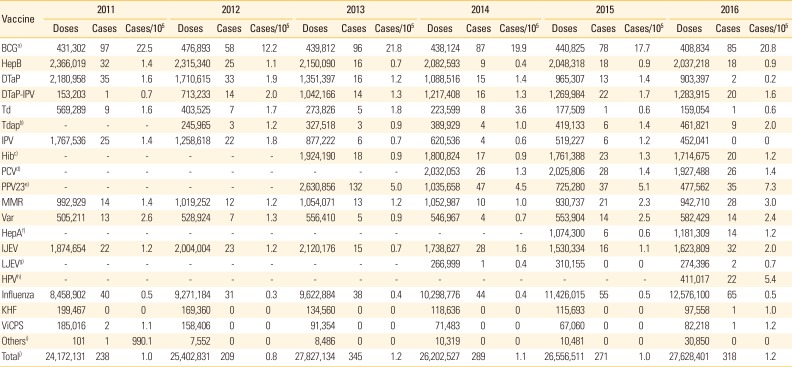
Number of reported adverse events and reporting rates by vaccine types
Table 3 shows the number of reported adverse events and the reporting rate (number of cases/100,000 doses) by vaccine type between 2011 and 2016. Overall, the annual AEFI reporting rates for all vaccinations registered from 2011 to 2016 ranged 0.8-1.2 (average, 1.1) cases/100,000 doses. The reporting rate of adverse events was highest for the BCG vaccine, followed by PPV23, and the human papillomavirus (HPV) vaccine in 2016. The BCG vaccine, including both intradermal and subcutaneous administration, had 58-97 reports of adverse events each year, and the average AEFI reporting rate for 6 years (19.0 cases/100,000 doses) was significantly higher than that of other vaccines. The AEFI reporting rate of PPV23, which was introduced in 2013, showed 7.3 cases in 2016 and average 5.2 cases/100,000 doses for four years. Since the introduction of the HPV vaccine in June 2016, 22 AEFI cases have been reported, with 5.4 cases/100,000 doses. The number of reported adverse reactions was second-highest following the influenza vaccination; however, the AEFI reporting rate for this vaccine (0.5 cases/100,000 doses) was low, due to the high number of doses.
Applications filed for injury compensation from 2011 to 2016
There were 515 applications filed for compensation over the 6-year period, including 29 cases of appeal and six cases of additional claims (
Table 2). In four cases, the final decision was deferred. Excluding duplicated cases of appeal and additional claims, as well as deferred cases, we reviewed 469 cases in total for this study. Out of these, 318 cases (67.8%) resulted in compensation and 151 cases (32.2%) resulted in dismissal. The highest number of applications filed for injury compensation (235 cases, 50.1%) involved the BCG vaccine (including its simultaneous inoculation with hepatitis B). There were 90 cases filed for the influenza vaccine, 55 cases filed for the PPV23 vaccine, 40 cases filed for the DTaP-IPV vaccine, and 20 cases filed for the Japanese encephalitis vaccine. The number of cases for each vaccine included simultaneous inoculation with other vaccines.
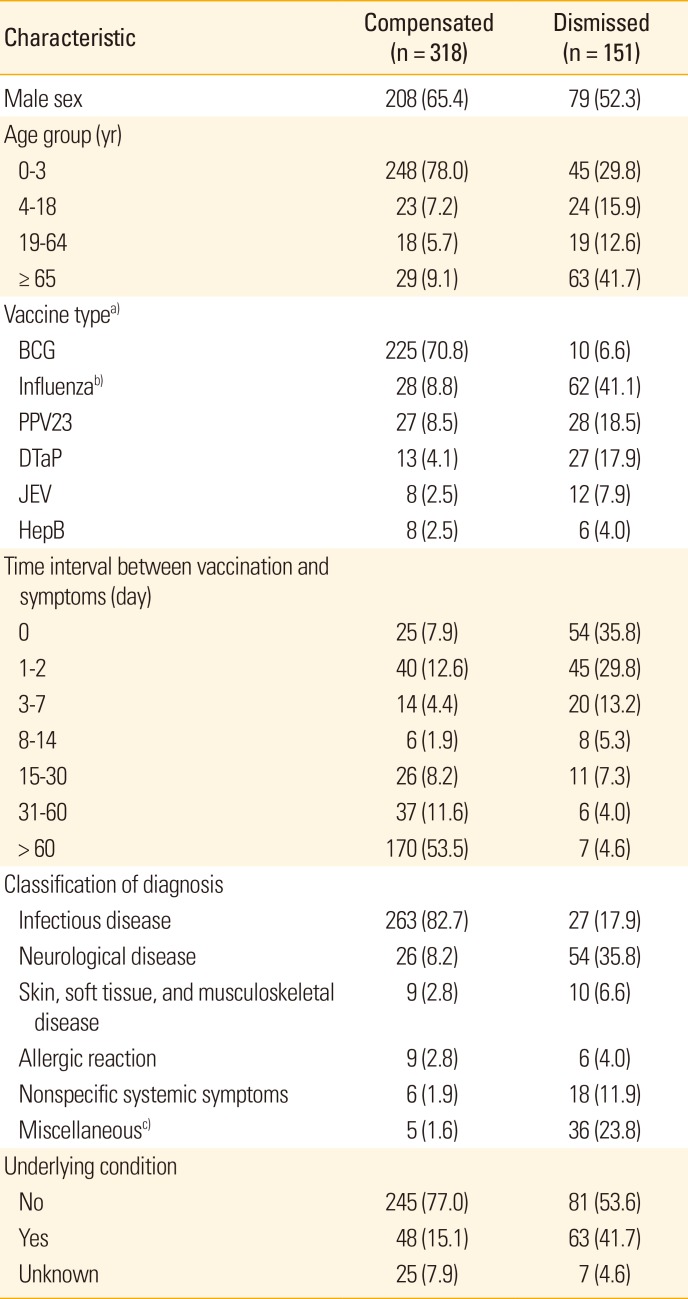
Table 4 compares the characteristics of the compensated claims and dismissed claims according to their injury compensation results. Several distinct differences exist between the compensated and the dismissed groups. The compensated group accounted for 71% of BCG vaccines, while the dismissed group accounted for 41% of influenza vaccines and 19% of PPV23 vaccines, respectively. The compensated group accounted for more than 80% of infections, including lymphadenitis and abscess due to the BCG vaccine. Additionally, this group accounted for four-fifths of affected infants under three year old, in line with characteristics of BCG vaccination. The majority of the adverse events in this group occurred more than 2 months after the inoculation, reflecting the characteristics of BCG lymphadenitis. In contrast, the dismissed group consisted of subjects in various age groups, including 42% of elderly people and 30% of children aged 3 years or younger. In particular, dismissed claims had noticeably shorter time intervals between vaccination and adverse event than did compensated claims.
Details of applications for injury compensation and results of deliberation
Among 235 applications filed for BCG vaccination, 225 cases (95.7%) were compensated. Compensation for the BCG vaccine accounted for 71% of the 318 compensated cases. Among the 225 cases of compensation for BCG-related adverse reaction, 217 cases (96.4%) reflected well-known adverse events, such as BCG lymphadenitis, ulcer or abscess formation. Four cases of BCG osteomyelitis and one case of disseminated BCG infection, soft tissue infection, infectious arthritis, and anaphylactic reactions were included.
Among 90 applications filed for influenza vaccine injury, 28 cases (31.1%) were approved for compensation. Of these, 18 cases concerned neurological diseases (nine cases of Guillain–Barré syndrome, six cases of encephalomyelitis, one case of peripheral neuropathy, one case of brachial plexus inflammation, and one case of narcolepsy), four cases concerned infections, and four cases concerned skin, soft tissue, and musculoskeletal diseases. Regarding age distribution, the elderly and adults comprised 80% of influenza-related cases. For PPV23-related cases, 27 of 55 applications (49.1%) were compensated, demonstrating a higher compensation ratio for PPV23 than for influenza. Regarding classification of adverse reaction type, 30 out of the 55 PPV23 cases were considered infections, 23 cases (76.7%) of which were compensated.
Discussion
KVICP is one of the best-organized, government-operated and government-funded, no-fault vaccine injury compensation systems in the world. According to a recent study, there are only 19 jurisdictions which have no-fault vaccine injury compensation system worldwide [
11]. Furthermore, the Korean government takes responsibility for evaluating causality of individual adverse events following vaccination. Very few previous studies have reviewed the reported AEFI and associated claims that were registered in our surveillance and compensation system; such relevant studies have presented only the total number of cases [
6712]. To our knowledge, this study constitutes the first research intended not only to present recently updated AEFI reports and claims, but also to review the details of reported serious AEFI, such as death and disability, and to analyze the results and characteristics of all filed claims registered in the KVICP.
Compared to other countries, Korea's annual AEFI reporting rates (average 1.1 cases/100,000 doses) were very low. According to a previous study [
7], fewer than 50 AEFI cases were reported between the establishment of the AEFI surveillance system and 2000. In 2005, when web-based reporting was introduced and patients or parents were allowed to report AEFI online [
5], the number of annual reported AEFI cases increased to over 300. In particular, the number of AEFI reports was as high as 2,380 and 741 cases in 2009 and 2010, respectively. These high reporting rates resulted from a surge in vaccinations due to a 2009 influenza pandemic. The AEFI reporting rate by vaccine type was highest for the BCG vaccine, with average 19.0 cases per 100,000 doses from 2011 to 2016, followed by the HPV and PPV23 vaccines, with average 5.4 and 5.2 cases, respectively, per 100,000 doses. However, careful interpretation of these data is critical. The AEFI reporting rate does not equal the actual proportion of AEFI occurrence. Because AEFI monitoring is a passive surveillance system, motivation to report AEFI, a particular interest in AEFI, or convenience of reporting AEFI might influence the number of events reported. The HPV vaccine was introduced to the NIP in June in 2016, yet public concerns about HPV vaccine safety persist in many countries, including South Korea [
1314]. Therefore, the KCDC actively informed public health centers regarding AEFI reporting as the NIP introduced the HPV vaccine, and actively investigated all reported AEFI cases for HPV. In addition, although the AEFI reporting rate was high for HPV, no claims were filed for compensation following HPV vaccination for 1 year. This means that few serious adverse events, such as hospital admission, have occurred following HPV vaccination. Therefore, we need more time to evaluate the precise safety of the HPV vaccine. Furthermore, public health centers and healthcare facilities that administer vaccines should inform vaccine recipients of common adverse reactions so that patients can respond appropriately.
Our low AEFI reporting rate might be resulted from our vaccine safety management system, which focuses primarily on the responses to serious AEFI and compensation for individual adverse events, rather than on AEFI surveillance and analysis among large populations. Precise and exact data regarding a reporting AEFI is required for call cases in our AEFI reporting system. We do not have simple and accumulated reporting system to monitor minor adverse reactions following immunization. Additionally, vaccine recipients or their parents can report adverse events through a website or over the telephone; however, these are not registered directly to our surveillance system, and public health center staff must review and register each case to our system. Therefore, some cases can be lost through this process due to lack of relevance or administrative fault. On the other hands, various stakeholders comprise the source of reported AEFI in the Vaccine Adverse Event Reporting System (VAERS) in the Unites States. Not only vaccine providers (37%) and vaccine recipients (10%), but also vaccine manufacturers (27%), report significant amounts of the total AEFI recorded in the system. The AEFI reporting rate was around 1,000 cases per 100,000 doses in VAERS [
15]. Since they receive AEFI reports from various sources, some cases could be erroneous, suggesting that the quality of our data may be superior to theirs [
16]. Therefore, we should not uniformly compare each country's system based solely on the aspect of reporting rate of AEFI. Our system is very effective for sustaining a high level of public trust in the NIP by responding rapidly to serious AEFI, and providing compensation for each serious adverse event resulting from immunizations recommended by the government. However, various mild AEFIs, which were not eligible for claims or academic review, were hardly detected and analyzed in our system. Therefore, we need a complementary system designed to monitor both mild and serious AEFIs, at least for newly introduced vaccines.
This study analyzed claims filed and compared compensated and dismissed claim groups by vaccine type, classification of adverse events, time interval between vaccination and symptoms, and other characteristics. The BCG vaccine comprised half of the total claims made and two-thirds of the total compensated cases. The adverse events following BCG vaccination, such as lymphadenitis and abscess or ulcer formation, are common, well-known, and accepted to have definite causal association with the BCG vaccine. The second-most common vaccine in terms of claims made was influenza. Compared to the BCG vaccine, various age groups and classifications of adverse reactions were included in influenza vaccine-related cases, and less than one-third received compensation. In particular, most non-specific systemic, gastrointestinal, or respiratory symptoms were dismissed. However, we cannot conclude the causality with only certain type of vaccine and adverse reaction. It is likely to acknowledge the causality between certain adverse events and vaccines if those events are common or accepted as possible adverse reactions as described in the literature, and if the time interval between vaccination and adverse reaction is appropriate. Additionally, a significant number (25 cases, 45%) of soft tissue infections, including cellulitis, resulted from PPV23-related cases. However, one-third of these subjects developed initial symptoms on the day of vaccination, which implies that some cases may not have resulted from inoculation site infection, but from another systemic inflammatory reaction to the antigen or vaccine ingredients [
17]. Further studies to review cellulitis-like adverse events following PPV23 in Korea should be conducted in order to prevent unnecessary antibiotic use and hospitalization.
It is very difficult to assess the causality between one clinical adverse event and an immunization. Thus, the WHO AEFI manual explains that the process of causality assessment for AEFI is to determine the likelihood of a causal association between the adverse reaction and the vaccines administered [
10]. Our assessment criteria have five categories following the previous WHO AEFI causality criteria [
9]. However, some cases cannot be classified into any of the five categories, because the causality association between certain adverse reactions and vaccines has not yet been determined by many studies. Because of this, the new WHO causality assessment manual introduced a new category, “indeterminate,” for adverse events that are neither consistent nor inconsistent in their causal association with immunization [
10]. While “indeterminate” is an acceptable and appropriate category in the view of science and academia, our goal is to determine whether to provide compensation for each case. In order to determine no-fault vaccine injury compensation, a connection must exist between the scientific causality assessment and the administrative measure. Therefore, it should be determined in advance, considering the objective of the KVICP, funding, and public trust of vaccine safety, whether to provide compensation when the causality for a filed case is indeterminate. It is also critical that new evidence of causality association from scientific and medical research be continuously monitored.
This study has some limitations. First, diagnosis of some adverse events could not be appropriately classified. Because all registered claims had their own individual diagnosis, instead of categorization by diagnostic codes, we classified claims according to the registered diagnosis without review of each case record. In addition, because skin, soft tissue, and musculoskeletal diseases, which had relatively low incidence, were arbitrarily categorized together, it was difficult to understand the correlation between certain types of adverse events and the vaccines received. Second, although we divided claims into compensated and dismissed groups in order to compare characteristics between the two groups, it is possible that the AEFI causality assessment could differ case-by-case, even if two people of the same age received the same type of vaccine and developed the same diagnosis of adverse event. Therefore, careful interpretation of the different characteristics between the two groups is required. Finally, we did not explain nor analyzed why results of causality assessments were determined, particularly in dismissed cases.
This study reviewed the reported AEFIs and related claims filed from 2011 to 2016, introduced the results of rapid responses to serious AEFIs during the same period, and compared the characteristics between compensated and dismissed cases. None of the 31 reported death cases during past six years showed causal association with a vaccine received under rapid response and evaluation. BCG vaccine comprised of the highest number of claims filed for compensation, followed by influenza and PPV23 vaccines. We have maintained national vaccine safety management system through both rapid responses to serious AEFI, and KVICP in order to sustain a high level of public trust in the NIP.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download