1. Singhi S, Kissoon N, Bansal A. Dengue and dengue hemorrhagic fever: management issues in an intensive care unit. J Pediatr (Rio J). 2007; 83(2 Suppl):S22–S35. PMID:
17530136.

2. Murray NE, Quam MB, Wilder-Smith A. Epidemiology of dengue: past, present and future prospects. Clin Epidemiol. 2013; 5:299–309. PMID:
23990732.
3. Kutsuna S, Kato Y, Moi ML, et al. Autochthonous dengue fever, Tokyo, Japan, 2014. Emerg Infect Dis. 2015; 21:517–520. PMID:
25695200.

4. Wilder-Smith A, Quam M, Sessions O, et al. The 2012 dengue outbreak in Madeira: exploring the origins. Euro Surveill. 2014; 19:20718. PMID:
24602277.

5. Lee SH, Nam KW, Jeong JY, et al. The effects of climate change and globalization on mosquito vectors: evidence from Jeju Island, South Korea on the potential for Asian tiger mosquito (Aedes albopictus) influxes and survival from Vietnam rather than Japan. PLoS One. 2013; 8:e68512. PMID:
23894312.

6. Capeding MR, Tran NH, Hadinegoro SR, et al. Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: a phase 3, randomised, observer-masked, placebo-controlled trial. Lancet. 2014; 384:1358–1365. PMID:
25018116.

7. Villar L, Dayan GH, Arredondo-Garcia JL, et al. Efficacy of a tetravalent dengue vaccine in children in Latin America. N Engl J Med. 2015; 372:113–123. PMID:
25365753.

8. Guy B, Briand O, Lang J, Saville M, Jackson N. Development of the Sanofi Pasteur tetravalent dengue vaccine: one more step forward. Vaccine. 2015; 33:7100–7111. PMID:
26475445.

9. Yung CF, Lee KS, Thein TL, et al. Dengue serotype-specific differences in clinical manifestation, laboratory parameters and risk of severe disease in adults, Singapore. Am J Trop Med Hyg. 2015; 92:999–1005. PMID:
25825386.

10. Liang H, Lee M, Jin X. Guiding dengue vaccine development using knowledge gained from the success of the yellow fever vaccine. Cell Mol Immunol. 2016; 13:36–46. PMID:
26435066.

11. Ferguson NM, Rodriguez-Barraquer I, Dorigatti I, Mier YT-RL, Laydon DJ, Cummings DA. Benefits and risks of the Sanofi-Pasteur dengue vaccine: Modeling optimal deployment. Science. 2016; 353:1033–1036. PMID:
27701113.

12. Bente DA, Rico-Hesse R. Models of dengue virus infection. Drug Discov Today Dis Models. 2006; 3:97–103. PMID:
18087566.

13. Velandia-Romero ML, Calderon-Pelaez MA, Castellanos JE. In vitro infection with dengue virus induces changes in the structure and function of the mouse brain endothelium. PLoS One. 2016; 11:e0157786. PMID:
27336851.

14. Guzman MG, Harris E. Dengue. Lancet. 2015; 385:453–465. PMID:
25230594.

15. St John AL. Influence of mast cells on dengue protective immunity and immune pathology. PLoS Pathog. 2013; 9:e1003783. PMID:
24367254.

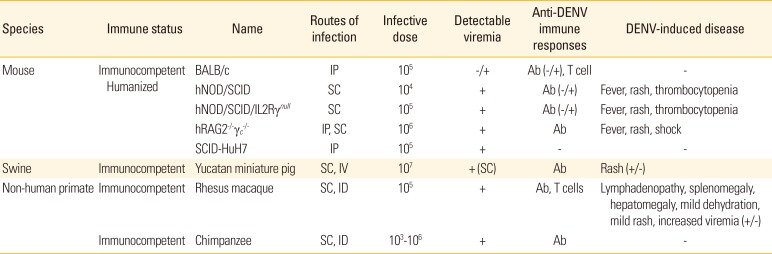
16. Bente DA, Melkus MW, Garcia JV, Rico-Hesse R. Dengue fever in humanized NOD/SCID mice. J Virol. 2005; 79:13797–13799. PMID:
16227299.

17. Mota J, Rico-Hesse R. Humanized mice show clinical signs of dengue fever according to infecting virus genotype. J Virol. 2009; 83:8638–8645. PMID:
19535452.

18. Sridharan A, Chen Q, Tang KF, Ooi EE, Hibberd ML, Chen J. Inhibition of megakaryocyte development in the bone marrow underlies dengue virus-induced thrombocytopenia in humanized mice. J Virol. 2013; 87:11648–11658. PMID:
23966397.

19. Wege AK, Melkus MW, Denton PW, Estes JD, Garcia JV. Functional and phenotypic characterization of the humanized BLT mouse model. Curr Top Microbiol Immunol. 2008; 324:149–165. PMID:
18481459.

20. Huang CY, Butrapet S, Pierro DJ, et al. Chimeric dengue type 2 (vaccine strain PDK-53)/dengue type 1 virus as a potential candidate dengue type 1 virus vaccine. J Virol. 2000; 74:3020–3028. PMID:
10708416.

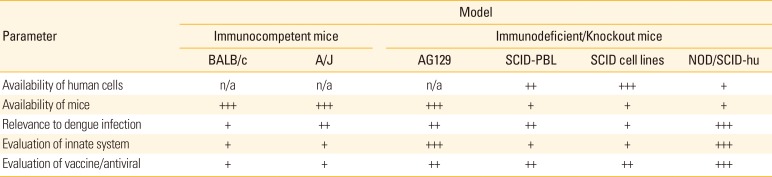
21. Shresta S, Sharar KL, Prigozhin DM, Snider HM, Beatty PR, Harris E. Critical roles for both STAT1-dependent and STAT1-independent pathways in the control of primary dengue virus infection in mice. J Immunol. 2005; 175:3946–3954. PMID:
16148142.

22. Chen J, Ng MM, Chu JJ. Molecular profiling of T-helper immune genes during dengue virus infection. Virol J. 2008; 5:165. PMID:
19117515.

23. Perry ST, Buck MD, Lada SM, Schindler C, Shresta S. STAT2 mediates innate immunity to Dengue virus in the absence of STAT1 via the type I interferon receptor. PLoS Pathog. 2011; 7:e1001297. PMID:
21379341.

24. Zust R, Toh YX, Valdes I, et al. Type I interferon signals in macrophages and dendritic cells control dengue virus infection: implications for a new mouse model to test dengue vaccines. J Virol. 2014; 88:7276–7285. PMID:
24741106.

25. Marchette NJ, Halstead SB, Falkler WA Jr, Stenhouse A, Nash D. Studies on the pathogenesis of dengue infection in monkeys. 3. Sequential distribution of virus in primary and heterologous infections. J Infect Dis. 1973; 128:23–30. PMID:
4198025.
26. Halstead SB, Shotwell H, Casals J. Studies on the pathogenesis of dengue infection in monkeys. I. Clinical laboratory responses to primary infection. J Infect Dis. 1973; 128:7–14. PMID:
4198027.

27. Halstead SB, Shotwell H, Casals J. Studies on the pathogenesis of dengue infection in monkeys. II. Clinical laboratory responses to heterologous infection. J Infect Dis. 1973; 128:15–22. PMID:
4198024.

28. Onlamoon N, Noisakran S, Hsiao HM, et al. Dengue virus-induced hemorrhage in a nonhuman primate model. Blood. 2010; 115:1823–1834. PMID:
20042723.

29. Goncalvez AP, Engle RE, St Claire M, Purcell RH, Lai CJ. Monoclonal antibody-mediated enhancement of dengue virus infection in vitro and in vivo and strategies for prevention. Proc Natl Acad Sci U S A. 2007; 104:9422–9427. PMID:
17517625.

30. Robinson JS. Yucatan miniature swine as an animal model for dengue-1 disease. Bethesda, MD: Uniformed Services University of The Health Sciences;2005.
31. Cassetti MC, Durbin A, Harris E, et al. Report of an NIAID workshop on dengue animal models. Vaccine. 2010; 28:4229–4234. PMID:
20434551.

32. Richter MK, da Silva Voorham JM, Torres Pedraza S, et al. Immature dengue virus is infectious in human immature dendritic cells via interaction with the receptor molecule DC-SIGN. PLoS One. 2014; 9:e98785. PMID:
24886790.

33. Tassaneetrithep B, Burgess TH, Granelli-Piperno A, et al. DC-SIGN (CD209) mediates dengue virus infection of human dendritic cells. J Exp Med. 2003; 197:823–829. PMID:
12682107.

34. Qu X, Che C, Gao A, et al. Association of Dectin-1 and DC-SIGN gene single nucleotide polymorphisms with fungal keratitis in the northern Han Chinese population. Mol Vis. 2015; 21:391–402. PMID:
25883525.
35. Miller JL, de Wet BJ, Martinez-Pomares L, et al. The mannose receptor mediates dengue virus infection of macrophages. PLoS Pathog. 2008; 4:e17. PMID:
18266465.

36. GlobalData. OpportunityAnalyzer: dengue vaccines: opportunity analysis and forecasts to 2020. London: GlobalData;2014.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download