Abstract
Purpose
Immunization against rabies in humans induces protective neutralizing antibodies; however, the induction of type 1 or type 2 cytokine mediated cellular immune responses following rabies vaccination is not understood. Hence, the present study investigated cellular cytokine responses in vaccinated individuals.
Materials and Methods
The study groups included healthy rabies antigen naive controls (n=10), individuals who received intradermal primary (n=10) or booster pre-exposure vaccination (n=20) and subjects who received postexposure rabies vaccination either by intradermal (n=18) or intramuscular (n=20) routes. The antigen specific cellular responses were analyzed by stimulating peripheral blood mononuclear cells with a rabies vaccine antigen in the interferon-γ (IFN-γ) and interleukin-4 (IL-4) enzyme-linked immunospot (ELISpot) assay. These responses were compared to the rabies virus neutralizing antibody (RVNA) titers that were measured by rapid fluorescent focus inhibition test.
Results
We observed that cellular and humoral immune responses to primary intradermal rabies vaccination could be greatly enhanced by a booster vaccine; and both type 1 and type 2 cytokine responses were significantly elevated. The magnitude of type 1 and type 2 cytokine responses did not differ significantly among the intramuscular and intradermal routes of postexposure vaccination. The number of cells producing IFN-γ and IL-4 correlated significantly with the levels of RVNA.
Conclusion
Both type 1 and type 2 cellular cytokine responses are strongly induced after rabies vaccination and directly correlate with levels of RVNA titers. The neutralizing antibody as well as the type 1 and type 2 cytokine responses may be important for vaccine induced protective responses against rabies.
Rabies is acute viral encephalitis caused by a highly neurotropic negative sense single stranded RNA virus belonging to the genus Lyssavirus of the family Rhabdoviridae. Though it is 100% fatal it is preventable by instituting timely pre-exposure or postexposure vaccination. Currently cell-culture derived vaccines are administered globally to provide immunity against rabies along with timely wound washing and local infiltration of rabies immune globulins [1]. In recent times a number of cell culture based rabies vaccines have been shown to possess long standing safety, immunogenicity and efficacy [2,3,4]. These vaccines are believed to induce strong humoral responses resulting in rabies virus neutralizing antibodies (RVNA) which neutralize the virus before it reaches the central nervous system (CNS) [5,6]. However, the role of rabies virus specific cell mediated immune responses are not yet clearly understood and may play a significant role in clearing the virus from the CNS [7]. Upon antigen encounter during viral infections, the naive CD4 T cells may either differentiate into a type 1 cytokine producing Th1 cells or type 2 cytokine producing Th2 cells, interleukin (IL)-17 secreting Th17 cells or follicular helper T (TFH) cells. The Th1 cells that are important for anti-viral immunity secrete type 1 panel of cytokines including interferon-γ (IFN-γ), IL-2, and tumor necrosis factor-α (TNF-α). These cells are known to promote interaction of CD8 T cells with dendritic cells and help B cells to produce high affinity and neutralizing antibodies [8,9]. The Th2 cells that secrete type 2 cytokines such as IL-4, IL-5, and IL-13 are known to be important for their helper activity to B cells for humoral immune responses; however, they are also known to inhibit protective responses and promote immunopathology during many viral infections [8,10]. There are a few studies which have addressed the induction of type 1 and type 2 cytokine responses following administration of viral vaccines and have shown that both arms of immune responses are induced after measles, hepatitis B, and influenza vaccines [11,12,13].
The immunogenicity and efficacy of cell culture derived anti-rabies vaccines have been evaluated generally by measuring the humoral responses by determining RVNA titers following vaccination by the standard intramuscular (IM) route. However, there is lack of knowledge with respect to type 1 and type 2 cellular cytokine responses following vaccination with cell culture rabies vaccines which are known to induce high levels of RVNA both by the IM and intradermal (ID) route. The World Health Organization (WHO) has recommended ID route of immunization for developing countries since 1992 [14]. In fact postexposure prophylaxis by ID vaccination could increase global supply of vaccine doses as well as reduce per person immunization cost [15]. In the last 3 decades, ID vaccination has been used extensively in some Asian countries thereby reducing the economic burden of rabies prophylaxis and contributing to a decline in the incidence of human rabies. We therefore wanted to determine if vaccination by ID route against rabies resulted in the induction of antigen specific cellular immune responses in addition to RVNA responses; whether an ID booster vaccine dose enhances rabies specific immune response and whether the route of immunization significantly affects these responses. Detection of cytokines such as IFN-γ, as a signature for type 1 response and IL-4 for the type 2 response, from antigen stimulated peripheral blood mononuclear cells (PBMCs) of vaccinated individuals is a valuable tool for analyzing cell mediated immune responses following vaccination. We hence undertook a study to compare the induction of the type 1 cytokine IFN-γ, and the type 2 cytokine IL-4, in PBMCs from individuals who received pre-exposure primary anti-rabies vaccination with or without booster vaccination by ID route and postexposure vaccination by either the ID or IM route. We have also attempted to analyze if these cellular responses correlate with serum RVNA titers.
A group of healthy subjects (n=10, 6 males, 4 females), who had no history of rabies vaccination or exposure to rabid animal bite, in the age group of 25-40 years, were included as negative controls. For the pre-exposure vaccination studies, we included healthy individuals in the age group of 27 to 58 years (n=30, 16 males, 14 females) who had received primary pre-exposure ID vaccine regimen six months earlier. A booster regimen was administered to a subgroup of these individuals (n=20) while the remaining subjects (n=10) were not given the booster. For the postexposure prophylaxis studies, individuals in the age group of 10 to 45 years who were administered postexposure IM vaccine (n=20, 15 males, 5 females) or postexposure ID vaccine (n=18, 12 males, 6 females) were included. The ID injections were given as per the updated Thai Red Cross (TRC) regimen [1] and the IM vaccine was administered using Essen regimen. The vaccine used for both groups was purified chick embryo cell vaccine (PCEC, Rabipur, Novartis Vaccines, Mumbai, India). The vaccination schedules followed for the different groups are listed in Table 1. The individuals who required postexposure prophylaxis approached our laboratory for assessment of the levels of neutralizing antibodies a week after the full course of vaccination.
A total of 6 mL blood was collected in EDTA Vacutainer tubes (BD, Becton Dickinson and Company, Franklin Lakes, NJ, USA) 7 days after the last dose of the priming vaccine or the booster, diluted with 6 mL of sterile phosphate buffered saline and overlaid on Histopaque gradient (Sigma, St. Louis, MO, USA). The samples were centrifuged at 450 g for 30 minutes, at 25℃ and the interface consisting of PBMCs was collected and washed in complete RPMI medium with 10% fetal calf serum (FCS), penicillin and streptomycin. PBMCs were frozen in liquid nitrogen. Serum or plasma was used for obtaining RVNA titers by rapid fluorescent focus inhibition test (RFFIT).
Enzyme-linked immunospot (ELISpot) assays for detecting IFN and IL-4 producing cells were performed with kits obtained from MABTECH (Nacka Strand, Sweden) by following instructions from the manufacturer. Micro well plates coated with anti-cytokine antibodies were blocked with complete RPMI medium containing 10% FCS. The frozen PBMCs were thawed and rested at 37℃ for one hour. The viable counts were obtained by trypan blue exclusion and PBMCs, 0.5×106, were added to each well for stimulation, in duplicates for each sample. Rabipur vaccine (Novartis, Mumbai, India) was used for stimulation of rabies specific IFN-γ and IL-4 cells at 10 µg/mL concentration.
Anti-CD28 antibody was added at 0.001 µg/mL. PBMCs from healthy individuals were stimulated with either PMA-ionomycin or anti-CD3 antibody as positive controls for the assay. The plates were incubated at 37℃ in a humidified incubator with 5% CO2 for 24 hours in case of IFN-γ and 40 hours for IL-4. The plates were then developed using biotinylated anti-cytokine monoclonal antibody, streptavidin alkaline phosphatase and BCIP substrate. Following appearance of spots the reaction was stopped by washing with distilled water and the plates were air dried before analysis on the ELISpot reader (AID, Strassberg, Germany). The non-specific background spots in unstimulated wells for each sample were subtracted from the antigen stimulated wells for obtaining antigen specific spot-forming cell (SFC) counts. The spot numbers in unstimulated wells typically ranged between zero and nine for IFN-γ and between two and ten for IL-4 ELISpots.
The RVNA titers were determined by RFFIT. The test was done as per WHO recommended procedure with some modifications [16]. We used BHK 21 (ATCC CCL 10) and 96 well tissue culture plates (Sigma) and BHK21 adapted CVS 11 strain of rabies virus. The reference serum used was an in-house serum calibrated against second international reference standard having a titer of 30 IU/mL (obtained from National Institute of Biological Standards, UK). Briefly, doubling dilutions of serum samples and reference serum (after heat inactivation at 56℃ for 30 minutes in a water bath) in duplicate were made in 96 well plates using Iscove's modified Dulbecco's media (IMDM; Sigma). To each 100 µL of serum dilution 100 µL of CVS (100 FFD50) was added and the plate to was incubated at 37℃ for 1 hour. A confluent monolayer of BHK 21 cells were trypsin treated and resuspended in 10 mL of IMDM with 10% FCS (Sigma). Cell control and virus controls were also included. To each well of the 96 well plates 100 µL of cell suspension was added and the plate was incubated at 37℃ in a CO2 incubator (Sanyo, Osaka, Japan). After 24 hours the cells were fixed in cold acetone for 30 minutes and stained by direct FAT using commercially available anti-rabies N-FITC conjugate (Light Diagnostics, Millipore, Temecula, CA, USA). The plates were then observed under an inverted fluorescence microscope (Nikon Eclipse TS100, Tokyo, Japan). The highest dilution of serum showing 50% inhibition of fluorescence foci was taken as end point dilution. The titer was converted to IU/mL in comparison with reference serum.
The data were analyzed using GraphPad Prism software version 5 (GraphPad, San Diego, CA, USA) and SPSS version 15.0 (SPSS Inc., Chicago, IL, USA). The T cell responses following ID vaccination were compared between non-boosted and boosted groups by Mann Whitney test. The antibody titers and the T cell responses between the IM and ID vaccinated groups were compared by the independent sample t-test. The correlation between RVNA titers and cellular cytokine responses were analyzed by the Pearson's correlation coefficient test. p-values of less than 0.05 were considered statistically significant.
The number of SFCs detected with PBMCs from healthy subjects who had no history of rabies vaccination or rabid animal bite was not higher than the assay background. On the other hand, SFCs for both IFN-γ and IL-4 were detectable in people who received pre-exposure rabies vaccination six months earlier (Figs. 1A, B, 2A, B).
Following stimulation of PBMCs with rabies vaccine antigen, the pre-exposure ID vaccinated group before a booster dose showed detectable but low levels of antigen specific IFN-γ and IL-4 responses indicating a waning immune response (Fig. 1A, B). However after 7 days following booster vaccination, we observed a significantly elevated IFN-γ (p<0.005) and IL-4 (p<0.0005) SFCs, suggesting that antigen specific responses were particularly enhanced due to a boosting effect (Figs. 1A, B, 2A, B). The results show that cellular responses were specifically enhanced due to a secondary response.
Among the individuals who received either the postexposure ID or IM vaccine regimens, there was a strong antigen specific IFN-γ and IL-4 response observed when analyzed seven days after vaccination with no statistically significant differences between the two groups for either of the cytokine producing cells (Fig. 3A, B).
The serum RVNA titers, as observed by the RFFIT assay, were at detectable levels six months after the primary ID vaccination, with all the ten individuals showing titers much higher than the 0.5 IU/mL which is regarded by the WHO as the adequate level following vaccination [1]. The RVNA titer increased significantly following a booster dose at six months as evidenced by the RFFIT assay at seven days following the booster vaccination (Fig. 1C).
The serum RVNA titers among the individuals receiving postexposure primary vaccination by either ID or IM route were highly elevated at seven days following the last dose. Interestingly, although individuals vaccinated by either of the routes showed high titers of RVNA, the group receiving IM vaccination showed significantly higher titers than those vaccinated by the ID route (p<0.005) (Fig. 3C).
It was interesting to note that both the type 1 and type 2 cytokine responses strongly correlated with the RVNA titers among individuals who received ID pre-exposure primary vaccination and those who received ID booster vaccination (Fig. 1D, E).
Since the introduction of CCV way back in 1975, the mainstay for assessing a successful vaccination has been to measure the levels of RVNA. This has also been the means for evaluating responses to different routes of vaccination as well as different brands of vaccines. The serum RVNA titer which is used as an indicator to evaluate the efficacy of rabies vaccines is believed to correlate with the protection offered by vaccines. Nevertheless the role of cellular immune responses in the induction of protective immunity against rabies is unclear. An earlier study that analyzed immune responses between 2 to 14 years after anti-rabies immunization reported significantly higher levels of neutralizing antibodies and proliferative responses in vaccinees as compared to control subjects [17]. In contrast, another study reported that vaccinated individuals with low antigen specific proliferative responses had high titers of neutralizing antibodies but no IFN-γ production and individuals with low titers of neutralizing antibodies showed high proliferative and IFN-γ responses [18]. Studies in animal models have shown that for effective clearance of virus from the CNS innate as well as adaptive T and B cell responses are required [19]. Indeed IFN-γ is also known to enhance production specific neutralizing antibody response in interaction with dendritic cells and B cells.
The main objective of the study was to know the extent of these responses following rabies vaccination and if there is any association between the route of vaccine administration and the immune response. Although we have used a highly purified chick embryo cell vaccine for vaccinations and as antigen for ELISpot assays, it is likely that some of the immune responses may be directed against other proteins which are part of the vaccine formulation. In this study we found a significant correlation between RVNA levels and antigen specific T cell response following both primary and booster immunization. Further, there was no significant difference in either type 1 or type 2 responses observed between the two routes of vaccination (IM and ID) which are currently recommended for immunization against rabies. There was a significant correlation between the RVNA titers and levels of cells producing both cytokines.
The cytokine producing T cells play a crucial role in both the generation and regulation of immune responses following vaccination. IFN-γ is critical for macrophage activation, T cell proliferation and differentiation, and up-regulation of antigen presenting proteins. Following vaccination with the Yellow Fever 17D vaccine virus IFN-γ is strongly induced at early stages in humans and monkeys; in the mouse model it is clearly shown to result in high levels of neutralizing IgG2a antibodies [20]. IFN-γ exerts an antiviral effect by promoting the lysis and clearance of virus-infected cells and by inhibiting viral gene expression and replication. In comparison, a Th2 response is characterized by IL-4 production and high level of rabies specific antibodies after vaccination which is traditionally considered as the hallmark of protective immunity against rabies infection. In our study, although we have not separated the CD4 to study antigen specific responses, since we are stimulating PBMCs with a soluble protein which will be predominantly processed by the exogenous pathway and presented on MHC II protein to CD4 T cells we assume that most of the cytokine producing cells are rabies specific CD4 T cells [21]. The positive correlation observed between numbers of IL-4 and IFN-γ producing rabies specific T cells and RVNA titers suggest that anti-rabies immunity is maintained by participation of both the IL-4 and IFN-γ.
In the pre-exposure group we found that after about six months following primary vaccination there is a gradual waning of the IFN-γ and IL4 producing cellular responses. However, following a single booster dose of the vaccine, we observed a striking enhancement in these responses as well as in the RVNA titers. This probably suggests the need for periodic booster doses of vaccination among people who are continuously at risk of rabies exposure.
In the postexposure group, we found high levels of RVNA titers seven days after the full course of vaccination by either ID or IM routes and there were strong type 1 and type 2 responses. Rabies vaccination may therefore prime strong humoral as well as cellular immune responses without skewing the response towards either type 1 or type 2. As the role of specific types of immune response in virus clearance from CNS is still not clear, it is probably advantageous to have balanced humoral and cellular responses after rabies vaccination specifically in situations where local infiltration of rabies immunoglobulins is not possible or when postexposure vaccination is delayed. Previously it has been shown that IFN gamma responses play a crucial role in prevention of measles and influenza [11,13] is pivotal in clearing intracellular virus.
To the best of our knowledge, this is the first study to analyze the induction of type 1 and type 2 cytokine responses in PBMCs following the administration of rabies vaccines. The results clearly indicate that a course of rabies vaccination, either pre-exposure or postexposure, stimulates both these types of cellular responses. The importance of type 1 and type 2 cytokine responses in contributing to the quality and duration of protective immunity against rabies needs to be further investigated. In the event of protective responses being compromised in the absence of one of these arms, it will be essential for anti-rabies vaccinations to elicit both types of T cells. We thereby suggest that due consideration be given to assessing these cell mediated responses while designing new schedules of vaccination or developing new rabies vaccines.
Figures and Tables
 | Fig. 1Enzyme-linked immunospot assay for rabies specific interferon-γ (IFN-γ) (A) and interleukin-4 (IL-4) (B) producing T cells in peripheral blood mononuclear cells (PBMCs), seven days after pre-exposure primary intradermal (ID) vaccination and after ID booster dose. (C) Rapid fluorescent focus inhibition test assay results for rabies virus neutralizing antibody (RVNA) titers in serum, seven days after primary ID vaccination and after ID booster dose. SFC, spot-forming cell. **p < 0.005 and ***p < 0.001 (Mann Whitney test). Pearson's correlation co-efficient analysis of antibody titres and cellular cytokine responses between naive, ID pre-exposure prime and boost groups. (D) RVNA versus IFN-γ. (E) RVNA versus IL-4. |
 | Fig. 2Representative image for enzyme-linked immunospot (ELISpot) responses from a healthy control, individual who received pre-exposure primary intradermal (ID) vaccination six months earlier and an individual who received booster ID vaccine at six months. The top row of wells represent the unstimulated peripheral blood mononuclear cell (PBMC) control wells while the bottom row represent the rabies antigen stimulated wells. The results from interferon-γ (A) and interleukin-4 (B) ELISpot assays are shown. |
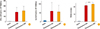 | Fig. 3Enzyme-linked immunospot assay for rabies specific interferon-γ (IFN-γ) (A) and interleukin-4 (IL-4) (B) producing T cells in peripheral blood mononuclear cells (PBMCs), seven days after postexposure primary intradermal (ID) and intramuscular (IM) vaccination. (C) Rapid fluorescent focus inhibition test assay for rabies virus neutralizing antibody (RVNA) titers in serum, seven days after postexposure primary ID and IM vaccination. ***p < 0.001 (Mann Whitney test). |
Table 1
Categories of the study population

References
1. World Health Organization. WHO technical report series, No. 982. WHO expert consultation on rabies. Second report. Geneva: World Health Organization;2013.
2. Briggs DJ, Mahendra BJ. Public health management of humans at risk. In : Jackson AC, Wunner WH, editors. Rabies. 2nd ed. Amsterdam: Elsevier Academic Press;2007. p. 545–566.
3. Madhusudana SN. Rabies. Gurgaon: Macmillan Medical Communications;2011. p. 148–158.
4. Ravish HS, Sudarshan MK, Madhusudana SN, et al. Assessing safety and immunogenicity of post-exposure prophylaxis following interchangeability of rabies vaccines in humans. Hum Vaccin Immunother. 2014; 10:1354–1358.

5. Flamand A, Raux H, Gaudin Y, Ruigrok RW. Mechanisms of rabies virus neutralization. Virology. 1993; 194:302–313.

6. Hooper DC, Morimoto K, Bette M, Weihe E, Koprowski H, Dietzschold B. Collaboration of antibody and inflammation in clearance of rabies virus from the central nervous system. J Virol. 1998; 72:3711–3719.

7. Johnson N, Cunningham AF, Fooks AR. The immune response to rabies virus infection and vaccination. Vaccine. 2010; 28:3896–3901.

8. Swain SL, McKinstry KK, Strutt TM. Expanding roles for CD4(+) T cells in immunity to viruses. Nat Rev Immunol. 2012; 12:136–148.

9. Sant AJ, McMichael A. Revealing the role of CD4(+) T cells in viral immunity. J Exp Med. 2012; 209:1391–1395.

10. Nair S, Bayer W, Ploquin MJ, Kassiotis G, Hasenkrug KJ, Dittmer U. Distinct roles of CD4+ T cell subpopulations in retroviral immunity: lessons from the Friend virus mouse model. Retrovirology. 2011; 8:76.
11. Gans HA, Yasukawa LL, Sung P, et al. Measles humoral and cell-mediated immunity in children aged 5-10 years after primary measles immunization administered at 6 or 9 months of age. J Infect Dis. 2013; 207:574–582.

12. Frazer IH, Jones B, Dimitrakakis M, Mackay IR. Intramuscular versus low-dose intradermal hepatitis B vaccine: assessment by humoral and cellular immune response to hepatitis B surface antigen. Med J Aust. 1987; 146:242–245.

13. Ghendon Y. The immune response to influenza vaccines. Acta Virol. 1990; 34:295–304.
14. Brown D, Fooks AR, Schweiger M. Using intradermal rabies vaccine to boost immunity in people with low rabies antibody levels. Adv Prev Med. 2011; 2011:601789.

15. Madhusudana SN, Mani RS. Intradermal vaccination for rabies prophylaxis: conceptualization, evolution, present status and future. Expert Rev Vaccines. 2014; 13:641–655.

16. Smith JS, Yager PA, Baer GM. A rapid fluorescent focus inhibition test (RFFIT) for determining rabies virus-neutralising antibody. In : Meslin FX, Kaplan MM, Koprowski H, editors. Laboratory techniques in rabies. 4th ed. Geneva: World Health Organization;1996. p. 181–191.
17. Thraenhart O, Kreuzfelder E, Hillebrandt M, et al. Long-term humoral and cellular immunity after vaccination with cell culture rabies vaccines in man. Clin Immunol Immunopathol. 1994; 71:287–292.

18. Moore SM, Hanlon CA. Rabies-specific antibodies: measuring surrogates of protection against a fatal disease. PLoS Negl Trop Dis. 2010; 4:e595.

19. Hooper DC, Roy A, Kean RB, Phares TW, Barkhouse DA. Therapeutic immune clearance of rabies virus from the CNS. Future Virol. 2011; 6:387–397.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download