Abstract
Purpose
Materials and Methods
Figures and Tables
Fig. 1
Development of recombinant fusion proteins. Recombinant fusion vectors were constructed as described in Materials and Methods. The map of the vectors and the DNA fragments of flaB, tetanus toxin fragment C (TTFC), and restriction enzyme sites are shown (A). Purified fusion proteins were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (B) and Western blot analysis (C). Putative structure for the fusion protein was predicted by bioinformatics tools (D). The model was constructed by the Phyre server (http://www.sbg.bio.ic.ac.uk/phyre/) using Protein Data Bank (PDB) data sets. TF, TTFC-FlaB fusion protein; FT, FlaB-TTFC fusion protein.
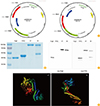
Fig. 2
Toll-like receptor 5 (TLR5)-mediated nuclear factor-κB (NF-κB) stimulating activity of the recombinant proteins. Fusion protein-mediated TLR5 signaling was determined by NF-κB-luciferase reporter assay. 293 cells were co-transfected with hTLR5 and pNF-κB-Luc and further treated with FlaB (100 ng/mL) or FlaB-tetanus toxin fragment C (FlaB-TTFC) (219 ng/mL) for 24 hours. Relative luciferase activities in cell extracts were analyzed by the dual-luciferase reporter assay system and normalized with pCMV-β-galactosidase as a control. ***p < 0.001.
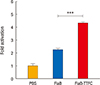
Fig. 3
Tetanus toxin fragment C (TTFC)-specific antibody responses after intranasal immunization. Mice were intranasally immunized with phosphate buffered saline (PBS), 1.5 µg TTFC alone (TTFC), 1.5 µg TTFC combined with 1.25 µg FlaB (T+F), or 2.75 µg FlaB-TTFC fusion protein (FT) three times at 1-week interval. One week after the last immunization, serum or mucosal samples were collected and TTFC-specific IgG (A) and IgA (B) titers were determined. Data are presented as the mean±SEM in each group. *p<0.05, **p<0.01, ***p<0.001 when compared with the T+F group and FT group.
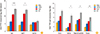
Fig. 4
Survival (A) and paralytic score (B) of mice challenged with tetanus toxin. Mice were intranasally immunized with phosphate buffered saline (PBS), 1.5 µg tetanus toxin fragment C alone (TTFC), 1.5 µg TTFC combined with 1.25 µg FlaB (T+F), or 2.75 µg FlaB-TTFC fusion protein (FT) three times at 1-week interval. One week after the last immunization, mice were subcutaneously challenged with 600× LD50 tetanus toxin. After the challenge, survival rate and tetanic phenotype were monitored for 7 days. Data represent the results from 7 mice in each experiment group. **p<0.01 compared with T+F group with FT group.

Table 1
Polymerase chain reaction primers used in this study

Table 2
Bacterial strains and plasmids used in this study

| Strain or plasmid | Characteristics | Source or reference |
|---|---|---|
| Escherichia coli | ||
| ER2566 | F- λ- fhuA2 [lon] ompT lacZ::T7 gene1 gal sulA11Δ(mcrC-mrr)114::IS10 R(mcr-73::miniTn10-TetS)2R(zgb-210::Tn10) (TetS) endA1 [dcm] | New England Biolabs Inc. |
| DH5α | F- φ80dlacZΔM15Δ(lacZYA-argF)U169 deoR recA1 endA1 hsdR17(rK- mK+) phoA supE44 λ- thi-gryA96 relA1 | Invitrogen |
| Plasmids | ||
| pCR2.1TOPO | Cloning vector; Apr; Kmr | Invitrogen |
| pCMM8209 | 1.3 kb NdeI-XhoI fragment containing ttfC cloned into pCR2.1TOPO | This study |
| pCMM8210 | 1.3 kb XhoI-PstI fragment containing ttfC cloned into pCR2.1TOPO | This study |
| pCMM8211 | 1.1 kb NdeI-XhoI fragment containing flaB cloned into pCR2.1TOPO | This study |
| pCMM8212 | 1.1 kb XhoI-PstI fragment containing flaB cloned into pCR2.1TOPO | This study |
| pTYB12 | N-terminal fusion expression vector in which the N-terminus of a target protein is a fused intein tag; Ampr | New England Biolabs, Inc. |
| pTETtac::ttfC | 1.3 kb DNA encoding the tetanus toxin fragment C cloned into pTETtac | [27] |
| pCMM250 | 1.5 kb EcoRI-PstI fragment containing ORF of flaB cloned into pTYB12 | [9] |
| pCMM8213 | 1.1 kb NdeI-XhoI fragment containing ORF of flaB cloned into pTYB12 | This study |
| pCMM8214 | 1.3 kb NdeI-XhoI fragment containing ttfC cloned into pTYB12 | This study |
| pCMM8215 | 2.5 kb NdeI-PstI fragment containing ttfC and flaB cloned into pTYB12 | This study |
| pCMM8216 | 2.5 kb NdeI-PstI fragment containing flaB and ttfC cloned into pTYB12 | This study |
Notes
We thank Dr. Annick Mercenier of Institut Pasteur de Lille for kindly providing the plasmid harboring TTFC. This study was supported by a grant from the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (No. A110983) and Chonnam National University Hospital Biomedical Research Institute (CRI 12022-1). We thank Vivek Verma for a careful reading of the manuscript.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download