Abstract
Purpose
The common triple therapy for Helicobacter pylori is challenged by the increasing cases of antibiotic resistant infections, raising the need to explore alternative therapies. Oral administration of egg yolk immunoglobulin Y (IgY) has been previously reported as a means of passive immunization therapy for H. pylori infections. In this work, we investigated the inhibitory effect of IgY on the attachment of H. pylori to AGS cell line.
Materials and Methods
Recombinant OipA was prepared. Hens were immunized with recombinant protein three times. IgY was purified from egg yolks of immunized hens using polyethylene glycol precipitation method. The inhibitory effect of the specific immunoglobulin was evaluated in AGS cell line infected with H. pylori.
Results
The presence of recombinant OipA (30 kD) was confirmed via sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Immunization of hens was confirmed using enzyme-linked immunosorbent assay. The purified IgY from egg yolks were assessed using SDS-PAGE and confirmed by western blot.
Helicobacter pylori is a spiral, gram negative, and microaerophilic bacterium that can colonize in gastric epithelial cells [1]. H. pylori is known as the main cause of chronic gastritis and is related to peptic ulcers and gastric cancer [2]. Although half of the world's population is infected with this bacterium, most of those infected are asymptomatic and 15%-20% of infected people develop the related diseases [3]. The most common treatment for H. pylori is triple therapy including the combination of two antibiotics (clarithromycin plus amoxicillin or metronidazole) with a proton pump inhibitor [4]. Increasing cases of antibiotic resistant H. pylori infections present a serious challenge in the treatment of infection [5]. Therefore it is necessary to explore alternative therapeutic approaches. Passive immunization with oral antibody can provide rapid protection against intestinal infections [67]. Recently, hen egg yolk immunoglobulin (IgY) has been reported to offer significant advantages compared to mammalian IgG, including cost-effectiveness, convenience, high yield and being non-invasive [8]. IgY, derived from immunized hen with whole-cell lysate of H. pylori, was shown to inhibit the growth of the bacteria and reduces gastric inflammation in infected Mongolian gerbils [9]. However, immunoglobulin against whole-cell lysates was reported to have cross-reaction with intestinal normal flora [10]. Therefore, it is essential to choose an appropriate virulent antigen of H. pylori for producing IgY. Approximately 4% of the H. pylori genome encodes for outer membrane proteins (OMPs); among them is the outer inflammatory protein (OipA), which is one of the major OMPs of the pathogen. OipA is an important virulence factor due to its role in adhesion to epithelial cells and increasing inflammation as the result of enhancing interleukin-8 secretion [1112]. Additionally, this inflammatory protein is thought to facilitate colonization and associate with other major virulent factor of H. pylori CagA, in development of gastric cancer [1213]. Due to its role in adherence and pathogenesis, specific antibody against OipA, may serve as an inhibitor of adhesion to gastric epithelial cell. In this study, specific IgY against the recombinant OipA protein produced and inhibitory effects of antibody against the H. pylori binding to AGS cell line was evaluated.
Expression and purification of recombinant OipA (rOipA) was performed according to our previous report [14]. Briefly, recombinant pET28a-oipA was transferred into Escherichia coli BL21 (DE3) strain (Novagene, Madison, WI, USA). Recombinant cells were grown in LB broth (Merck, Darmstadt, Germany), which contained 30 µg/mL kanamycin (Sigma, St. Louis, MO, USA) to OD620 of 0.1. Expression of recombinant protein was induced with 1 mmol/L of IPTG (Thermo Scientific, Waltham, MA, USA). Three-hour induced cells were collected (8,000 ×g/4℃) and were washed with phosphate buffered saline (PBS; pH 7.4). Purification of rOipA was carried via Ni2+- chelate chromatography according to our previous report in hybrid method of denaturation on column resolubilization [15], and was assessed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and NanoDrop (Wilmington, DE, USA) (data not shown).
Ten 25-week-old white Leghorn hens were obtained from laboratory animal production center of Pasteur Institute of Iran (Alborz, Karaj, Iran). Hens were immunized by 20 µg/mL of rOipA formulated with complete Freund's adjuvant intramuscularly administrated into two sides of chest area (0.5 mL to each side). Two subsequent boosters containing rOipA formulated with incomplete Freund's adjuvant free rOipA respectively were administered with two weeks of interval. A control group which received PBS (pH 7.2) intramuscularly was considered. One month after immunization, hens were bled from sub-wing vein and serum samples were collected. Serum immunoglobulin titers specifically directed to rOipA were measured via enzyme-linked immunosorbent assay (ELISA), and hen with the highest titration of antibody was chosen for egg collection. Eggs from selected hens were collected daily for one month and were stored at 4℃.
ELISA test was performed according to a previous report with some modifications [16]. 96-well ELISA plates (Nunc, Copenhagen, Denmark) were coated with 10 µg/mL of recombinant OipA diluted in PBS and were incubated overnight at 4℃. After three times of washing (PBS containing 0.05% Tween 20; PBS-T), plates were blocked with 1% bovine serum albumin at 37℃ for 1 hour. After three washing, 1:200 dilution of each serum sample was added to the first well and was diluted subsequently (1:2). Plates were incubated at 37℃ for 60 minutes. Plates were washed and incubated for 1 hour at 37℃ with 100 µL horseradish peroxide (HRP)-conjugated rabbit anti-IgY antibody (Sigma, Seelz, Germany) in the ratio of 1:5,000. Finally, plates were washed and 100 µL TMB was added to each well. Chromogenic reaction was allowed to be developed for 15 minutes at 25℃ until the reaction was stopped by adding 50 µL of 1 M H2SO4; optical density of wells were recorded at 450 nm.
Purification of IgY was carried out by polyethylene glycol (PEG) precipitation method [17]. Briefly, the yolk was carefully separated from egg white. PBS was added to egg yolk by two volume and mixed; thereafter PEG 6000 was added by 3.5% of the total volume and was mixed by rotation for 10 minutes. Mixture was centrifuged at 10,000 ×g (4℃, 20 minutes) and supernatant was passed through a filter-paper, PEG 6000 was added to the filtrate by 8.5% w/v, and was mixed properly for 10 minutes; followed by centrifugation as described above. The pellet was carefully dissolved in 1 mL PBS (pH 7.2) by means of a glass sticks and vortex. PBS was added to a final volume of 10 mL. PEG 6000 was added to the solution by 12% w/v, mixed by rotation and centrifuged as described above. The pellet was dissolved carefully in 800 µL PBS. The final solution was dialyzed overnight against 50 volumes normal saline 0.1% (1,600 mL) with 3 exchanges. Purified IgY-OipA was analyzed by SDS-PAGE and western blot, subsequently [1819].
H. pylori SS1 was used for attachment studies. H. pylori was cultured on specific medium composed of brucella agar (QUELAB, Montreal, Canada) supplemented with 5% fetal bovine serum (FBS; Gibco, Paisley, UK), 10% de-fibrinated sheep blood and antibiotics (amphotericin B, 2.5 µg/mL; vancomycin, 10 µg/mL trimethoprim, 5 µg/mL; and polymyxin B, 2.5 IU/mL; Sigma, Seelze, Germany). Inoculated plates were incubated in microaerophilic condition for 72 hours (37℃, 10% CO2) [9].
AGS cell line was obtained from Pasteur Institute of Iran (Tehran, Iran). AGS cells were grown in tissue culture flasks (Jet-Biofil, Elgin, IL, USA) as described before [20]. The cells were grown in RPMI 1640 medium (Gibco) supplemented with FBS 10%, penicillin 100 IU/mL, and streptomycin 100 µg/mL (Gibco), and were incubated in a humidified atmosphere supplemented with 5% CO2 at 37℃.
Co-culture was performed by procedure that was described by Yu et al. [21] and Kim et al. [22] with some modification. Briefly, AGS cells were cultured in 96-well polypropylene tissue culture plates (Jet-Biofil) and H. pylori SS1 was cultured as explained before. Bacterial cells were washed off the plate aseptically and were re-suspended in antibiotic-free RPMI 1640 medium to an OD600nm of 0.5. Various concentrations of IgY-OipA were added as triplicate to each bacterial suspension followed by 2 hours of incubation under microaerophilic conditions while rotating. The treatment was done for IgY obtained from control group as well. AGS monolayer was washed with sterile PBS twice and 200 µL of IgY-treated and non-treated bacterial suspension was added at an H. pylori/AGS ratio of 100:1. Intact AGS cells were considered as negative controls. Cultures were incubated for 3 hours. Co-cultures were washed with PBS twice to remove any unattached bacteria; cells were fixed by adding 300 µL of pure methanol for 15 minutes. Methanol was poured off gently and plates were let to be completely dried in 37℃ for 1 hour three times. H. pylori cells were traced via ELISA using rabbit anti H. pylori whole-cell lysate (produced in our laboratory) by 1:2,000 dilution and detection HRP-conjugated anti-Rabbit IgG (Sigma) by 1:4,000, then developed with TMB. Reactions were stopped with 1 M H2SO4 and OD450nm of wells were recorded.
The ELISA results for the blood serum obtained from immunized hens subjected to the rOipA injection showed significantly high affinity toward recombinant protein. The ELISA result of hen with the highest antibody response was showed in Fig. 2.
Purification from egg yolks were carried out and yielded a total of 35 mg purified IgY-OipA per egg; purified IgY was stored at -20℃. SDS-PAGE showed a light chain (25 kD) and heavy chain (60 kD) (Fig. 3). To analysis of specificity of produced IgY against rOipA, western blotting was performed (Fig. 4). The result was shown that IgY was able to recognize rOipA and a single band (30 kD) was detected.
H. pylori SS1 suspensions which were treated with different concentration of IgY-OipA showed a various result in inhibition of bacterial attachment to the AGS cells (Fig. 5). As depicted, 1 mg/mL and 0.5 mg/mL concentrations of the IgY-OipA showed the optimal inhibitory effects towards the attachment of the pathogen to AGS cells.
Increasing antibiotic resistance among H. pylori poses a significant challenge for clinicians and has been prohibited in European countries since 2006 [8]. Passive immunization is an attractive alternative procedure for prevention and therapy of H. pylori infections. IgY is one of the candidates for oral (passive) immunization approach and has the important advantage of not being toxigenic. Since it does not activate mammalian complement and does not intract with mammalian Fc receptor, IgY does not cause inflammatory response [6]. Oral administration of specific IgY can be effective against a variety of intestinal pathogens incuding Salmonella, rotavirus [8232425]. There are numerous reports on the feasibility of using IgY-supplement food [26]. IgY is also more convenient in terms of production compared to mammalian IgG, including high yield, economical production, non-invasive method, simple and fast isolation, and non-toxigenic usage in diet [82728].
IgY is produced either against whole cell lysates or against cell components. IgY produced against whole-cell lysates of bacteria has been reported to decrease colonization of H. pylori in infected Mongolian gerbils; however, efficiency and specificity of the antibody decreased due to the possibility of cross-reaction with other bacteria, including human gastrointestinal tract [7910]. Urease is the only selective antigen reported for H. pylori. This enzyme neutralizes the acidic gastric environment by converting urea to ammonia ions, and can help H. pylori survive in the stomach [29]. Suzuki et al. [30] investigated the effect of dietary anti-urease IgY on H. pylori infection. Although they declared a decline of H. pylori associated gastritis and partial attenuation of gastric urease activity, they could not demonstrate the role of produced IgY in preventing of H. pylori colonization. In other study, Nomura et al. [7] demonstrated attenuation of gastric mucosal inflammation in H. pylori infected gerbils without affecting the level of bacterial colonization. However, Malekshahi et al. [10] reported prevention of bacterial colonization in a similar study.
In our study, recombinant OipA was isolated from H. pylori and was used to induce production of anti-OipA IgY which was obtained from immunized hens. Bacterial adhesins are considered as important antigens since they aid in the colonization of H. pylori on gastric epithelial cells [29]. OipA is a major H. pylori adhesin and plays a major role in the bacterial colonization of the gastric mucosa in animal models [123132]. Dossumbekova et al. [12] showed that OipA mutagenesis could result in decreasing bacterial adhesion to gastric epithelial in vitro.
We used AGS cell line as a model for gastric epithelium to study the effect of specific IgY on bacterial attachment to epithelial cell, due to difficulty in obtaining primary gastric cells [33]. IgY-OipA produced in this study effectively inhibited the attachment of H. pylori to the AGS cells. No change in bacterial attachment was observed when IgY obtained from the control group of hens was used for the treatment. Furthermore, our work demosntrates the simlpicity and cost effectiveness of the procedure due to the high yield of the produced IgY compared to mammalian IgG, which proves that IgY as an economical source of anti-adhesion for passive therapy. The yield of antibody obtained from PEG percipitation method in our study was 35 mg per egg or 13 mg/mL of egg yolk. This was higher volume than the obtained from other IgY purification methods; for instance, 9.8 mg IgY/mL egg yolk was routinely obtained from water dilution method reported by Akita and Nakai [34]. The yield of IgY purified by ion exchange chromatography method was also too low [35]. In another study, Shin et al. [9], attained 9.4 mg of IgY per mL of egg yolk using modified Akita and Nakai method. Amount as much as 8.9 mg IgY/mL of yolk was reported by Hodek et al. [36] using filtaration followed by precipitation of IgY with sodium chloride.
In conclution, the observed specific inhibitory effect of the IgY-OipA, proposes OipA as a potential antigen for producing potent antibodies for passive therapy. Our results suggest IgY-OipA to be a deserving candidate for further investigations on alternative therapeutic or prophylaxis agents.
Figures and Tables
 | Fig. 1Detection and purification of expressed OipA. (A) OipA was detected by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Lane M: low molecular weight standard protein size marker (kDa); lane 1: non-induced OipA; lane 2, 3, 4, and 5: induced OipA with 1 mmol/L IPTG at t = 0, 1, 2, and 3 hr, respectively. 30-kD band were detected in induced lanes. T = 3 hr was the best time for induction. (B) Purified OipA was detected using SDS-PAGE. Lane M: low molecular weight standard protein size marker (kDa); lane 1: 30-kD band was detected as purified OipA. |
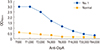 | Fig. 2The reaction between IgY-OipA produced in serum and recombinant OipA. The titration of serum in the first hen having the highest affinity toward recombinant protein was shown (No. 1). Serum of non-immunized hens (phosphate buffered saline injected) were used as negative control. |
 | Fig. 3Purified IgY detection by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). After purification by polyethylene glycol precipitation method, IgY was detected by SDS-PAGE. Lane IgY: purified IgY; light chain (25 kDa) and heavy chain (60 kDa); M: low molecular weight standard protein size marker (kDa). |
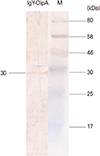 | Fig. 4Specific IgY against rOipA detection by western blot analysis. Lane IgY-OipA: rOipA band (30 kDa) was detected; lane M: low molecular weight standard protein size marker (kDa). Western blot analysis using IgY against OipA showed a single 30-kDa protein on nitrocellulose paper. Purified IgY was used as first layer, and rabbit-anti-IgY conjugated with horseradish peroxide enzyme was used as second-layer antibody. |
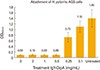 | Fig. 5Inhibition of Helicobacter pylori attachment to AGS cell line by IgY-OipA. Attahment of H. pylori SS1 to AGS cells were assessed by co-culture and enzyme-linked immunosorbent assay. Treatments of bacteria with ≥ 0.5 mg/mL concentration of IgY-OipA inhibited attachment of bacteria to the AGS cell line. |
References
1. Ricci V, Romano M, Boquet P. Molecular cross-talk between Helicobacter pylori and human gastric mucosa. World J Gastroenterol. 2011; 17:1383–1399.

2. Matsunari O, Shiota S, Suzuki R, et al. Association between Helicobacter pylori virulence factors and gastroduodenal diseases in Okinawa, Japan. J Clin Microbiol. 2012; 50:876–883.

3. Liu YE, Gong YH, Sun LP, Xu Q, Yuan Y. The relationship between H. pylori virulence genotypes and gastric diseases. Pol J Microbiol. 2012; 61:147–150.

4. Gisbert JP, Pajares JM. Treatment of Helicobacter pylori infection: the past and the future. Eur J Intern Med. 2010; 21:357–359.

5. Gerrits MM, van Vliet AH, Kuipers EJ, Kusters JG. Helicobacter pylori and antimicrobial resistance: molecular mechanisms and clinical implications. Lancet Infect Dis. 2006; 6:699–709.

6. Kovacs-Nolan J, Mine Y. Egg yolk antibodies for passive immunity. Annu Rev Food Sci Technol. 2012; 3:163–182.

7. Nomura S, Suzuki H, Masaoka T, et al. Effect of dietary anti-urease immunoglobulin Y on Helicobacter pylori infection in Mongolian gerbils. Helicobacter. 2005; 10:43–52.

8. Xu Y, Li X, Jin L, et al. Application of chicken egg yolk immunoglobulins in the control of terrestrial and aquatic animal diseases: a review. Biotechnol Adv. 2011; 29:860–868.

9. Shin JH, Yang M, Nam SW, et al. Use of egg yolk-derived immunoglobulin as an alternative to antibiotic treatment for control of Helicobacter pylori infection. Clin Diagn Lab Immunol. 2002; 9:1061–1066.

10. Malekshahi ZV, Gargari SL, Rasooli I, Ebrahimizadeh W. Treatment of Helicobacter pylori infection in mice with oral administration of egg yolk-driven anti-UreC immunoglobulin. Microb Pathog. 2011; 51:366–372.

11. Kudo T, Nurgalieva ZZ, Conner ME, et al. Correlation between Helicobacter pylori OipA protein expression and oipA gene switch status. J Clin Microbiol. 2004; 42:2279–2281.

12. Dossumbekova A, Prinz C, Mages J, et al. Helicobacter pylori HopH (OipA) and bacterial pathogenicity: genetic and functional genomic analysis of hopH gene polymorphisms. J Infect Dis. 2006; 194:1346–1355.

13. Franco AT, Johnston E, Krishna U, et al. Regulation of gastric carcinogenesis by Helicobacter pylori virulence factors. Cancer Res. 2008; 68:379–387.

14. Teymournejad O, Mobarez AM, Hassan ZM, Noori S, Moazzeni SM, Khoramabadi N. Cloning, expression, purification and toxicity evaluation of Helicobacter pylori outer inflammatory protein A. Indian J Microbiol. 2013; 53:391–394.

15. Aghababa H, Mohabati Mobarez A, Khoramabadi N, et al. A comparative approach to strategies for cloning, expression, and purification of Mycobacterium tuberculosis mycolyl transferase 85B and evaluation of immune responses in BALB/c mice. Mol Biotechnol. 2014; 56:487–497.

16. Khabiri AR, Bagheri F, Alimohammadian MH, Assmar M, Nadaf SR. Leishmanin skin test in guinea pig with a single purified protein of Leishmania major. Exp Parasitol. 2005; 111:239–243.

17. Pauly D, Chacana PA, Calzado EG, Brembs B, Schade R. IgY technology: extraction of chicken antibodies from egg yolk by polyethylene glycol (PEG) precipitation. J Vis Exp. 2011; (51):3084.

18. Ramezani V, Vatanara A, Najafabadi AR, Shokrgozar MA, Khabiri A, Seyedabadi M. A comparative study on the physicochemical and biological stability of IgG1 and monoclonal antibodies during spray drying process. Daru. 2014; 22:31.

19. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970; 227:680–685.

20. Zhang ZW, Dorrell N, Wren BW, Farthingt MJ. Helicobacter pylori adherence to gastric epithelial cells: a role for non-adhesin virulence genes. J Med Microbiol. 2002; 51:495–502.

21. Yu CC, Yang JC, Chang YC, et al. VCP phosphorylation-dependent interaction partners prevent apoptosis in Helicobacter pylori-infected gastric epithelial cells. PLoS One. 2013; 8:e55724.

22. Kim N, Park WY, Kim JM, et al. Analysis of gene expression profile of AGS cells stimulated by Helicobacter pylori adhesion. Gut Liver. 2007; 1:40–48.

23. Schade R, Terzolo HR. IgY-technology: application and trends. EPC 2006. 12th European Poultry Conference; 2006 Sep 10-14; Verona, Italy. Beekbergen: World's Poultry Science Association (WPSA);2006.
24. Lee EN, Sunwoo HH, Menninen K, Sim JS. In vitro studies of chicken egg yolk antibody (IgY) against Salmonella enteritidis and Salmonella typhimurium. Poult Sci. 2002; 81:632–641.

25. Sarker SA, Casswall TH, Juneja LR, et al. Randomized, placebo-controlled, clinical trial of hyperimmunized chicken egg yolk immunoglobulin in children with rotavirus diarrhea. J Pediatr Gastroenterol Nutr. 2001; 32:19–25.

26. Schade R, Zhang XY, Terzolo HR. Use of IgY antibodies in human and veterinary medicine. In : Huopalahti R, Lopez-Fandino R, Anton M, Schade R, editors. Bioactive egg compounds. . Berlin: Springer-Verlag;2007. p. 213–222.
27. Rahman S, Van Nguyen S, Icatlo FC Jr, Umeda K, Kodama Y. Oral passive IgY-based immunotherapeutics: a novel solution for prevention and treatment of alimentary tract diseases. Hum Vaccin Immunother. 2013; 9:1039–1048.
28. Tini M, Jewell UR, Camenisch G, Chilov D, Gassmann M. Generation and application of chicken egg-yolk antibodies. Comp Biochem Physiol A Mol Integr Physiol. 2002; 131:569–574.

29. Andersen LP. Colonization and infection by Helicobacter pylori in humans. Helicobacter. 2007; 12:Suppl 2. 12–15.

30. Suzuki H, Nomura S, Masaoka T, et al. Effect of dietary anti-Helicobacter pylori-urease immunoglobulin Y on Helicobacter pylori infection. Aliment Pharmacol Ther. 2004; 20:Suppl 1. 185–192.

31. Yamaoka Y, Kita M, Kodama T, et al. Helicobacter pylori infection in mice: role of outer membrane proteins in colonization and inflammation. Gastroenterology. 2002; 123:1992–2004.

32. Yamaoka Y. Mechanisms of disease: Helicobacter pylori virulence factors. Nat Rev Gastroenterol Hepatol. 2010; 7:629–641.

33. Keates AC, Tummala S, Peek RM Jr, et al. Helicobacter pylori infection stimulates plasminogen activator inhibitor 1 production by gastric epithelial cells. Infect Immun. 2008; 76:3992–3999.

34. Akita EM, Nakai S. Comparison of four purification methods for the production of immunoglobulins from eggs laid by hens immunized with an enterotoxigenic E. coli strain. J Immunol Methods. 1993; 160:207–214.

35. Ko KY, Ahn DU. Preparation of immunoglobulin Y from egg yolk using ammonium sulfate precipitation and ion exchange chromatography. Poult Sci. 2007; 86:400–407.

36. Hodek P, Trefil P, Simunek J, Simunek J, Hudecek J, Stiborova M. Optimized protocol of chicken antibody (IgY) purification providing electrophoretically homogenous preparations. Int J Electrochem Sci. 2013; 8:113–124.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download