Abstract
Advantages of DNA vaccination against infectious diseases over more classical immunization methods include the possibilities for rapid manufacture, fast adaptation to newly emerging pathogens and high stability at ambient temperatures. In addition, upon DNA immunization the antigen is produced by the cells of the vaccinated individual, which leads to activation of both cellular and humoral immune responses due to antigen presentation via MHC I and MHC II molecules. However, so far DNA vaccines have shown most efficient immunogenicity mainly in small rodent models, whereas in larger animals including humans there is still the need to improve effectiveness. This is mostly due to inefficient delivery of the DNA plasmid into cells and nuclei. Here, we discuss technologies used to overcome this problem, including physical means such as in vivo electroporation and co-administration of adjuvants. Several of these methods have already entered clinical testing in humans.
The first reporting showing that the direct injection of naked plasmid DNA carrying eukaryotic genes into the mammalian muscle leads to endogenous expression of and to a specific immune response against the encoded protein were published some 25 years ago and provided the basis for the development of DNA-vaccines [1,2,3]. These experiments DNA-based immunization has been developed further into a promising tool for the fight against many important challenges to human and animal health, including infectious diseases, cancer or allergy. The advantages of this technology over existing methods include safety (the plasmids used are non-replicating in eukaryotic cells), the possibility to stimulate potent cellular immune responses (due to MHC I-mediated presentation of the antigen which is made by the transfected cells), rapid adaptation to antigenic variants (by simple cloning techniques), simple production systems (amplification and purification in Escherichia coli is uncomplicated and relatively cheap), the possibility of combinatory vaccines (only by mixing different DNA molecules [4]), and the potential to be used in settings devoid of a cold chain (due to the high stability of DNA).
DNA vaccines usually consist of DNA plasmids, which express antigens following their transfer into a vaccinee. They thereby mediate the endogenous production of a foreign protein, including its natural conformation and appropriate post-translational modifications. This is of major importance since endogenous expression appears to be favorable for the induction of neutralizing antibodies and a balanced cellular immune response. In this context, DNA immunization has been shown to be able to induce potent Th1-mediated cellular immune responses, which is different to existing techniques such as inactivated pathogens or recombinant subunit vaccination [5]. Such advantages are commonly accepted and underlined by the fact that some DNA vaccines are already licensed in the veterinary sector. In addition to serving as a vaccination platform on its own, DNA immunization was also shown to induce powerful priming immune responses in combination with other vaccine techniques as booster immunizations, such as viral vectors, recombinant proteins or virus-like particles.
Until now DNA vaccines have only been licensed in the veterinary sector, including one application as immune therapy for melanoma in dogs (Oncept), a vaccine for the prevention of rhabdovirus disease in fish (Apex-IHN), and a West Nile virus (WNV) vaccine for horses (West-Nile-Innovator). The forth DNA plasmid licensed is not a vaccine, but it encodes the growth hormone releasing factor for breeding sows and is licensed for the food production industry resulting in more alive piglets in their litters and higher weight of these piglets [6]. The WNV-DNA vaccine has been tested in mice, birds and horses. Interestingly, the vaccine induced striking protective immune responses after a single application of DNA electroporation (EP) device from Genetronic Inc. (now: Inovio Inc.) of 100 µg or even 0.1 µg DNA in mice measured by intraperitoneal and mosquito challenge [7]. However, horses were immunized only by intramuscular application of 1 mg DNA in 1 mL phosphate buffered saline without EP, since horses appear to be intolerant to electric pulses. The absence of an uptake enhancement might be the reason of the low immune responses in the horses after DNA vaccination [7]. The same WNV DNA vaccine was experimentally applied in a variety of bird species using different formulations and delivery methods [8,9,10]. The first genetic DNA vaccination on the market however was against the infectious hematopoietic necrosis virus (IHNV) in the rainbow trout [11]. In a later DNA vaccine study Sockeye salmon with a mean weight of 150 g were injected with 25 µg of naked DNA resulting in high neutralizing antibody titers. In this study also Rainbow trout with mean weight of 2 g were immunized by intramuscular injection of 1, 5, or 10 µg DNA vaccine resulting in a nearly complete survival after challenge with IHNV in all vaccinated groups [12]. The immunotherapeutic DNA vaccine for dogs was licensed in 2010 to treat malignant melanoma. The application showed effective antibody responses and prolonged survival. The DNA was transferred intramuscularly by needle free injection (Biojector 2000) with of a total of four vaccinations in 2-week intervals ranging between 100 and 1,500 µg per dose [13].
However, despite these licensed veterinary applications, DNA vaccination is still facing limitations in immunogenicity, which have until today prevented its use on a global scale, most importantly in humans. Promising results from small rodent models were hardly seen in larger species including non-human primates or humans. In the following we will discuss methods which aim to overcome these limitations by increasing immunogenicity of DNA vaccines against infectious diseases. Several of these strategies are currently being used in clinical trials to develop the first DNA vaccines for the use in humans.
In the last few years several studies have been published on DNA sensing by cytosolic proteins, and the understanding of the innate immune mechanisms triggered by recognition of DNA is increasing. The inflammatory signal upon cytosolic DNA recognition is adjuvanting the DNA vaccination per se via the activation of two major types of proinflammatory pathways. These sensor molecules for cytosolic DNA identified so far include AIM2, IFI16, DDX41, and cGAS [14,15,16,17,18]. The interplay of these recognition molecules is orchestrated by a major key molecule which transfers the signal to the innate immune response: stimulator of interferon gene (STING) [19,20]. It is very likely that the understanding of the mechanisms of DNA recognition by the sensor molecules and of the signal-delivery to STING, which initiates an interferon (IFN) response will in future improve the usage of DNA as vaccine.
However, in order to on the one hand strongly trigger such innate immune mechanisms and on the other hand ensure an optimal expression of the antigen, it is essential that DNA is efficiently delivered into the cells and transferred into the nuclei [21]. As a consequence, the inefficient transfer of plasmid DNA into mammalian cells and nuclei in vivo is still one of the major obstacles in DNA vaccinology. A striking difference of the immunogenicity after transfer of naked DNA was observed when small rodent models were compared to larger animal species, especially non-human primates. This difference was thereafter named "simian barrier" because many DNA studies in non-human prinates and the first human clinical trials were carried out without or with only little induction of immune response [22,23,24]. The reasons for the lack of reproducibility of many results obtained in mice after the applications in larger animals are still not fully understood. Possible explanations may be differences in the ratio of applied DNA versus body weight or differences in the DNA-uptake of target cells. A large number of different strategies are being employed to overcome these problems (reviewed in Kutzler and Weiner [25]). In early studies bombardment with gold particles was used to increase the delivery efficiency of plasmid DNA [26]. Since then sophisticated physical DNA delivery methods have become an important area for vaccine research. In vivo EP combines the injection of DNA with electric pulses into the side of injection (Fig. 1) [24], and different in vivo EP technologies have been analyzed during the last years [27]. Currently intramuscular and intradermal EP are the predominant technologies used to deliver DNA vaccines in clinical trials. Several cell types were used for the analysis of in vivo uptake efficiency. Most of them express the transferred plasmids only for a few days [28]. In contrast, mature muscle cells express the plasmid-encoded protein for months [29,30]. Therefore, to date intramuscular DNA EP has proven the most effective delivery strategy [31]. In contrast, intradermal DNA vaccination is leading to immunogenicity most likely because of the high presence of antigen-presenting cells in the skin. These cells include Langerhans cells in the epidermis and dendritic cells in the dermis [32,33].
Other physical DNA-delivery technologies include the aforementioned bombardment via gold particles (gene gun) [34,35] jet stream DNA injection (Biojector 2000) [35,36], intradermal EP using different devices [37,38,39,40], plate applicator for transcutaneous EP [41], and DNA tattooing [23,41,42,43]. Hence, until today a variety of different methods for the delivery of DNA vaccines have been developed. However, no side-by-side investigations were performed to compare immunogenicity and efficacy of these different technologies, in fact most of the vaccine studies only compare one newly established device to a control group and not to other devices. Therefore we have analyzed in an non-human primates the immunogenicity of a DNA encoding the fusion protein of the respiratory syncytial virus delivered by intramuscular EP, intradermal EP, DNA-tattooing or by intramuscular injection without any adjuvants [41]. In this study, we showed that the humoral immune response was induced to high levels by immunization via both intramuscular and intradermal EP and DNA-tattooing. In contrast, only the intramuscular EP induced convincing systemic cellular responses to the vaccine antigen. Moreover, the induction of mucosal T-cell responses was polyfunctional only in an additional group which received an adenoviral boost expressing the same antigen [41].
Adjuvants are a powerful technique to enhance the immunogenicity of vaccines. They are part of several vaccines, especially those containing inactivated pathogens or protein subunit antigens. The overall principle is the enhancement of immune responses due to mechanisms such as causing a local inflammatory response, the direct induction of specific cytokines or the slow release of antigen from antigen-adjuvant complexes. Two major groups of adjuvants are being tested together with DNA vaccines. First, the "classical" ones, i.e., chemical compounds that are also used with established vaccine technologies. Secondly, "genetic adjuvants;" i.e., proteins encoded by the same or another DNA plasmid. In contrast, compounds which enhance the immunogenicity by optimizing the delivery of the DNA and thereby antigen expression are not counted as adjuvants here, because their effect on the immune system is not a direct one.
When using classical adjuvants together with DNA vaccines, several aspects are different to using adjuvants with established vaccine technologies. For example, in many cases the adjuvant is mixed with the antigen before administration of the vaccine. This enables a physical interaction of antigen and adjuvant, which in many cases generates a "depot-effect" upon application, i.e., the slow release of the antigen and longer interaction with the immune cells. In contrast, the plasmid of DNA vaccines is not the physical antigen but its coding sequence, and as a consequence no such direct interaction can be generated. In addition, when antigen and adjuvant are co-injected, both come into contact with the immune system at the same place and at the same time. However, after DNA-application, there is a lag phase of several hours to days, which is caused by the time needed for the transfected cells to produce sufficient amounts of antigens for stimulation of the immune system. Since many adjuvants work immediately after application, their effect might already have passed its peak or even disappeared at the time the antigen is present. On the other hand, plasmid DNA is usually produced in bacteria and therefore contains unmethylated CpG motives. These have an adjuvant effect by themselves via stimulating the innate immune system through Toll-like receptor (TLR) 9 [44], which could even be enhanced by adding additional CpG sequences into the plasmid [45].
Several chemical adjuvants have been tested together with DNA vaccines, however, most studies were performed in small rodent models [46,47,48,49,50]. Since in mice DNA vaccines are usually quite immunogenic and do not need a significant enhancement by adjuvants, these data obtained are not directly transferable to larger animal species. This is exemplified by studies with aluminium salts (also referred to as alum), probably the most widely used chemical adjuvant which is also licensed for the use in humans. The effect of alum on DNA vaccination was systematically studied [51,52]. A clear increase in humoral immune responses was seen with aluminium phosphate in mice. However, in non-human primates the differences to the non-adjuvanted controls were far less pronounced, especially when compared to the effect of alum on a recombinant protein antigen in the same animal species [51,53]. The positive effect of alum in mice was attributed to the boosting of the immune response elicited by the antigen expressed, hence not to a direct interaction with alum and the DNA plasmid [51]. In larger animals such as large birds or dogs, alum have so far been used in a few DNA vaccine trials [54,55,56]. There were no control groups without the adjuvant included in these studies, hence it is not possible to judge whether there was any benefit of alum. However, in a study of the WNV DNA vaccine in American crows no difference in immunogenicity was observed between birds immunized with or without aluminium phosphate [10]. Likewise, in humans, aluminium phosphate showed no effect on the performance of a human immunodeficiency virus (HIV) type 1 (HIV-1) DNA vaccine [57]. Therefore, it seems that, at least in larger animal species, alum do not work when co-injected with DNA vaccines to the same extend as they do with recombinant antigens. Alum are detectable for months at the injection sites [58,59], which could indicate that the failure of alum as adjuvant for DNA vaccines is not due to the slow appearance of DNA-delivered antigens, but rather to the lack of a direct interaction between adjuvant and antigen before injection.
An adjuvant that was developed to boost particularly DNA immunizations is the cationic lipid formulation Vaxfectin [60]. Although cationic lipids are known to efficiently deliver DNA to cells, the adjuvant effect of Vaxfectin is not induced by enhancing the in vivo transfection efficiency. In contrast, a direct modulation of immune pathways by the compound seems to be more crucial for the increase in immunogenicity [61]. The adjuvant is also being tested with other vaccine techniques, such as inactivated pathogens [14,15,16,17,18,62]. Vaxfectin has been successfully used together with a variety of DNA vaccines in small animal models [63,64,65]. In non-human primates, it significantly enhanced the antibody response to a DNA vaccine against measles virus, whereas there was no effect on virus-specific IFN-γ producing T-cells [66]. The adjuvant has also been tested with an influenza DNA vaccine in a human phase I trial and was well tolerated [67]. However, due to the absence of a non-adjuvanted control group, the effect of Vaxfectin on the human immune response could not be determined. A clinical phase I study with a DNA vaccine against dengue virus including such a control group is currently running (NCT 01502358).
The WNV DNA vaccine licensed for use in horses was formulated with MetaStim, an oil-in-water emulsion [68], but it was not published whether the adjuvant had an effect on immunogenicity. The TLR3-agonist poly ICLC, a stabilized poly I:C analogue, was proven a successful adjuvant with protein vaccines in mice [69]. However, no effect was seen in combination with a simian immunodeficiency virus DNA vaccine in rhesus macaques [70].
Taken together, several chemical adjuvants have been used with DNA vaccines in large animals and humans so far, but the actual effect of the adjuvants were not investigated in all of these studies. For the majority of chemical adjuvants under development, their usefulness in DNA vaccination needs to be addressed in more detail, especially by using animal models different from rodents.
Instead of using chemical compounds, which lead to the stimulation of certain cytokines, these cytokines can also be delivered directly with the DNA vaccine, either on the same or on a separate expression plasmid. This enables the appearance of the cytokine at the same time and in the same area as the vaccine antigen. The effects of plasmids encoding cytokines such as interleukin (IL)-10, IL-12, or IFN-γ together with DNA vaccines have been studied in a variety of animal and disease models, up to clinical trials in humans, and in many cases significant improvements in immunogenicity have been achieved (recently reviewed by Flingai et al. [71].
In addition to the co-delivery of cytokine genes, an increasing number of studies describe the usage of plasmids coding for immune signaling molecules, either as partial or as full-length genes. Many adjuvants function by activating the innate immune system via binding to TLRs. An alternative to using TLR ligands is the expression of proteins which mediate signaling directly downstream of activated TLRs. This was tested by incorporating two genes, MyD88 and TRIF, into DNA-vaccine plasmids against influenza or rabies [72,73]. Both genetic adjuvants were able to enhance the resulting immune responses.
Another innate immune mechanism, which is being explored for improving DNA vaccination is the sensing of viral infections via the two pathogen recognition receptors RIG-1 and MDA-5. Both proteins detect the presence of viral RNA in the cytosol. Co-delivery of the coding sequence of MDA-5 on a vaccine plasmid against influenza in chicken resulted in significantly higher antibody titers as compared to the non-adjuvanted controls. The genetic adjuvant also led to increased protection of the animals in a H5N1 challenge [74]. Similarly, the protein virus-induced signaling adapter (also named mitochondrial antiviral-signaling protein), which is activated by RIG-I, was able to enhance cellular immune responses to an influenza DNA vaccine [75]. Co-expression of an RNA-based RIG-I agonist led to an improved antibody avidity after DNA vaccination with an influenza hemagglutinin-coding plasmid [76].
Other strategies for genetic adjuvants include components of the complement system, protein aggregation domains, chemokines, or co-stimulatory molecules [50,77,78].
Hence, whereas DNA plasmids encoding certain cytokines have already entered clinical testing in humans, studies with many other genetic adjuvants were mostly performed in mice. Therefore, it remains to be seen to what extent these promising results can be transformed into powerful strategies to boost DNA vaccines in larger animals and humans.
Besides several clinical studies of DNA immunization in the context of cancer and autoimmune disease in humans, DNA vaccines have also been clinically tested against infectious viral diseases, most prominently HIV, hepatitis C virus, and cytomegalovirus (CMV). Initially one criticism for the usage of DNA vaccines in humans was the possibility of stable integration of DNA into the genome, which could lead to unwanted side effects even up to oncogenesis. However, so far several studies have demonstrated that the rate of DNA integration in vivo is actually lower than the rate of spontaneous mutagenesis [79]. In addition, no negative effects such as induction of autoimmunity or transfer of antibiotic resistance markers could be demonstrated. At present hundreds of volunteers have already received DNA vaccines without any known significant adverse event. However, the limitations as stand-alone platform for the induction of immune responses were also apparent in many studies completed to date. Nevertheless, until now several clinical trials with DNA vaccines delivered by intradermal or intramuscular EP have been performed and have shown both humoral and cellular immune responses which persists for several years [80,81].
One recent trial, HVTN 505, was a phase 2 study enrolling around 2,200 individuals to test the safety and efficacy of a biojector-delivered intramuscular DNA prime followed by an adenovirus vector serotype 5 (Ad5) boost vaccination [82]. The outcome was comparable to that observed after the STEP study published in 2008 [83], namely that the vaccinated individuals had a higher probability of HIV infection than the placebo group (reviewed by Odondo [84]). Hence, although clearly immune responses were elicited in the vaccine group these immune responses could apparently not protect against the virus infection. Whether this is an effect of reverting the protective towards a more susceptible immune response via "enhancer CD4+ cells" or a suppressive effect of the immune response is still under discussion [85,86].
In another HIV DNA vaccine clinical trail the level of HIV specific immune responses was increased 70-fold by using intramuscular in vivo EP as compared to intramuscular delivery alone [87]. A subsequent analysis showed that the antibody responses were directed towards the V2 loop of HIV env which was previously shown to correlate with protective efficacy [88,89].
In addition to these results in the HIV field, several trials were performed to study DNA vaccine technologies for the prevention of other infection diseases. One clinical trial phase 3 is currently starting to determine the ability of the therapeutic CMV vaccine TransVax. This DNA is a bivalent vaccine that contains plasmids encoding tegument phosphoprotein 65 and the surface glycoprotein B of CMV, to produce protective immune responses and provide clinical benefit in CMV-seropositive recipients for hematopoietic stem-cell transplantations. The plasmids are formulated with poloxamer CRL 1005 and with benzalkonium chloride, compounds which enhance the delivery of DNA [90]. The phase 2 trial showed that the DNA-vaccine was safe and well tolerated. Furthermore the vaccine significantly reduced the occurrence of viremia and prolonged the time to viremic episodes compared to the placebo group in the follow-up [91].
Many studies with DNA vaccines showed that by using viral vectors as heterologous boost vaccination, immunogenicity could be increased [92] and this consequently led to heterologous prime-boost strategies in clinical trials. Vectors employed for these developments include modified vaccinia Ankara (MVA) [93,94,95], NYVAC [96], fowlpox [97], and canarypox (ALVAC) viruses [98] as well as adenoviral vectors [82,99,100].
A lot of effort has been put into the improvement of DNA vaccines. Several clinical trials involving sophisticated delivery technologies, including DNA EP, are currently being executed. It will be of highest interest whether the results obtained point towards clear improvements in immunogenicity and protection.
It also seems very important to transfer results obtained with one antigen/target disease to other applications. A DNA vaccine delivered via one method can be very immunogenic but still fail in protecting against a certain infection, due to factors such as escape mutants or intrinsic requirements for immunity. Nevertheless, a DNA vaccine delivered with the same method can be very protective against another disease. Therefore, crosstalk between researchers in the field of DNA vaccinology seems essential to push DNA vaccine development further and transform a promising technology into real vaccines.
Figures and Tables
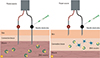
Fig. 1
Different electroporation delivery devices. Schematic view on DNA immunization by intramuscular and intradermal electroporation. (A) For intramuscular electroporation an array of needle electrodes carry an electrical current to the cells in the muscle layer. After an electronic pulse the cell membrane of muscle cells is temporarily permeable, allowing DNA plasmid to enter the cell. (B) For the intradermal electroporation the needle electrodes are placed on or introduced into the skin. The DNA plasmids are taken up by the dendritic cells (Langerhans cells) of the skin upon the electronic pulses.
References
1. Wolff JA, Malone RW, Williams P, et al. Direct gene transfer into mouse muscle in vivo. Science. 1990; 247:1465–1468.

2. Ulmer JB, Donnelly JJ, Parker SE, et al. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993; 259:1745–1749.

3. Jiao S, Williams P, Berg RK, et al. Direct gene transfer into nonhuman primate myofibers in vivo. Hum Gene Ther. 1992; 3:21–33.

4. Stab V, Nitsche S, Niezold T, et al. Protective efficacy and immunogenicity of a combinatory DNA vaccine against influenza A virus and the respiratory syncytial virus. PLoS One. 2013; 8:e72217.

5. Liu MA. DNA vaccines: an historical perspective and view to the future. Immunol Rev. 2011; 239:62–84.

6. Khan AS, Bodles-Brakhop AM, Fiorotto ML, Draghia-Akli R. Effects of maternal plasmid GHRH treatment on offspring growth. Vaccine. 2010; 28:1905–1910.

7. Davis BS, Chang GJ, Cropp B, et al. West Nile virus recombinant DNA vaccine protects mouse and horse from virus challenge and expresses in vitro a noninfectious recombinant antigen that can be used in enzyme-linked immunosorbent assays. J Virol. 2001; 75:4040–4047.

8. Kilpatrick AM, Dupuis AP, Chang GJ, Kramer LD. DNA vaccination of American robins (Turdus migratorius) against West Nile virus. Vector Borne Zoonotic Dis. 2010; 10:377–380.

9. Turell MJ, Bunning M, Ludwig GV, et al. DNA vaccine for West Nile virus infection in fish crows (Corvus ossifragus). Emerg Infect Dis. 2003; 9:1077–1081.

10. Bunning ML, Fox PE, Bowen RA, et al. DNA vaccination of the American crow (Corvus brachyrhynchos) provides partial protection against lethal challenge with West Nile virus. Avian Dis. 2007; 51:573–577.

11. Anderson ED, Mourich DV, Fahrenkrug SC, LaPatra S, Shepherd J, Leong JA. Genetic immunization of rainbow trout (Oncorhynchus mykiss) against infectious hematopoietic necrosis virus. Mol Mar Biol Biotechnol. 1996; 5:114–122.
12. Corbeil S, Lapatra SE, Anderson ED, et al. Evaluation of the protective immunogenicity of the N, P, M, NV and G proteins of infectious hematopoietic necrosis virus in rainbow trout oncorhynchus mykiss using DNA vaccines. Dis Aquat Organ. 1999; 39:29–36.

13. Bergman PJ, McKnight J, Novosad A, et al. Long-term survival of dogs with advanced malignant melanoma after DNA vaccination with xenogeneic human tyrosinase: a phase I trial. Clin Cancer Res. 2003; 9:1284–1290.
14. Burckstummer T, Baumann C, Bluml S, et al. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat Immunol. 2009; 10:266–272.

15. Hornung V, Ablasser A, Charrel-Dennis M, et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009; 458:514–518.

16. Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009; 458:509–513.

17. Unterholzner L, Keating SE, Baran M, et al. IFI16 is an innate immune sensor for intracellular DNA. Nat Immunol. 2010; 11:997–1004.

18. Stetson DB, Medzhitov R. Recognition of cytosolic DNA activates an IRF3-dependent innate immune response. Immunity. 2006; 24:93–103.

20. Bhat N, Fitzgerald KA. Recognition of cytosolic DNA by cGAS and other STING-dependent sensors. Eur J Immunol. 2014; 44:634–640.

21. Lechardeur D, Lukacs GL. Intracellular barriers to non-viral gene transfer. Curr Gene Ther. 2002; 2:183–194.

22. Otten G, Schaefer M, Doe B, et al. Enhancement of DNA vaccine potency in rhesus macaques by electroporation. Vaccine. 2004; 22:2489–2493.

23. Verstrepen BE, Bins AD, Rollier CS, et al. Improved HIV-1 specific T-cell responses by short-interval DNA tattooing as compared to intramuscular immunization in non-human primates. Vaccine. 2008; 26:3346–3351.

24. Widera G, Austin M, Rabussay D, et al. Increased DNA vaccine delivery and immunogenicity by electroporation in vivo. J Immunol. 2000; 164:4635–4640.

26. Tang DC, DeVit M, Johnston SA. Genetic immunization is a simple method for eliciting an immune response. Nature. 1992; 356:152–154.

27. Luckay A, Sidhu MK, Kjeken R, et al. Effect of plasmid DNA vaccine design and in vivo electroporation on the resulting vaccine-specific immune responses in rhesus macaques. J Virol. 2007; 81:5257–5269.

28. Fazio VM. "Naked" DNA transfer technology for genetic vaccination against infectious disease. Res Virol. 1997; 148:101–108.

29. Hartikka J, Bozoukova V, Jones D, et al. Sodium phosphate enhances plasmid DNA expression in vivo. Gene Ther. 2000; 7:1171–1182.

30. Satkauskas S, Bureau MF, Mahfoudi A, Mir LM. Slow accumulation of plasmid in muscle cells: supporting evidence for a mechanism of DNA uptake by receptor-mediated endocytosis. Mol Ther. 2001; 4:317–323.

31. March JB. Modern vaccine adjuvants and delivery systems: second international conference. Expert Rev Vaccines. 2006; 5:753–759.

32. Kanitakis J. Anatomy, histology and immunohistochemistry of normal human skin. Eur J Dermatol. 2002; 12:390–399.
33. Mathers AR, Larregina AT. Professional antigen-presenting cells of the skin. Immunol Res. 2006; 36:127–136.

34. Klinman DM, Conover J, Bloom ET, Weiss W. Immunogenicity and efficacy of a DNA vaccine in aged mice. J Gerontol A Biol Sci Med Sci. 1998; 53:B281–B286.

36. Bahloul C, Taieb D, Diouani MF, et al. Field trials of a very potent rabies DNA vaccine which induced long lasting virus neutralizing antibodies and protection in dogs in experimental conditions. Vaccine. 2006; 24:1063–1072.

37. Maruyama H, Ataka K, Higuchi N, Sakamoto F, Gejyo F, Miyazaki J. Skin-targeted gene transfer using in vivo electroporation. Gene Ther. 2001; 8:1808–1812.

38. Heller LC, Jaroszeski MJ, Coppola D, McCray AN, Hickey J, Heller R. Optimization of cutaneous electrically mediated plasmid DNA delivery using novel electrode. Gene Ther. 2007; 14:275–280.

39. Hooper JW, Golden JW, Ferro AM, King AD. Smallpox DNA vaccine delivered by novel skin electroporation device protects mice against intranasal poxvirus challenge. Vaccine. 2007; 25:1814–1823.

40. Hirao LA, Wu L, Khan AS, Satishchandran A, Draghia-Akli R, Weiner DB. Intradermal/subcutaneous immunization by electroporation improves plasmid vaccine delivery and potency in pigs and rhesus macaques. Vaccine. 2008; 26:440–448.

41. Grunwald T, Tenbusch M, Schulte R, et al. Novel vaccine regimen elicits strong airway immune responses and control of respiratory syncytial virus in nonhuman primates. J Virol. 2014; 88:3997–4007.

42. Bins AD, Jorritsma A, Wolkers MC, et al. A rapid and potent DNA vaccination strategy defined by in vivo monitoring of antigen expression. Nat Med. 2005; 11:899–904.

43. Pokorna D, Rubio I, Muller M. DNA-vaccination via tattooing induces stronger humoral and cellular immune responses than intramuscular delivery supported by molecular adjuvants. Genet Vaccines Ther. 2008; 6:4.

44. Hemmi H, Takeuchi O, Kawai T, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000; 408:740–745.

45. Zhang A, Jin H, Zhang F, et al. Effects of multiple copies of CpG on DNA vaccination. DNA Cell Biol. 2005; 24:292–298.

46. Jayakumar A, Castilho TM, Park E, Goldsmith-Pestana K, Blackwell JM, McMahon-Pratt D. TLR1/2 activation during heterologous prime-boost vaccination (DNA-MVA) enhances CD8+ T Cell responses providing protection against Leishmania (Viannia). PLoS Negl Trop Dis. 2011; 5:e1204.

47. Luo Z, Shi H, Zhang H, et al. Plasmid DNA containing multiple CpG motifs triggers a strong immune response to hepatitis B surface antigen when combined with incomplete Freund's adjuvant but not aluminum hydroxide. Mol Med Rep. 2012; 6:1309–1314.

48. Jin H, Li Y, Ma Z, et al. Effect of chemical adjuvants on DNA vaccination. Vaccine. 2004; 22:2925–2935.

49. Chuang CM, Monie A, Hung CF, Wu TC. Treatment with imiquimod enhances antitumor immunity induced by therapeutic HPV DNA vaccination. J Biomed Sci. 2010; 17:32.

50. Saade F, Petrovsky N. Technologies for enhanced efficacy of DNA vaccines. Expert Rev Vaccines. 2012; 11:189–209.

51. Ulmer JB, DeWitt CM, Chastain M, et al. Enhancement of DNA vaccine potency using conventional aluminum adjuvants. Vaccine. 1999; 18:18–28.

52. Wang S, Liu X, Fisher K, et al. Enhanced type I immune response to a hepatitis B DNA vaccine by formulation with calcium- or aluminum phosphate. Vaccine. 2000; 18:1227–1235.

53. Ruiz W, McClements WL, Jansen KU, Esser MT. Kinetics and isotype profile of antibody responses in rhesus macaques induced following vaccination with HPV 6, 11, 16 and 18 L1-virus-like particles formulated with or without Merck aluminum adjuvant. J Immune Based Ther Vaccines. 2005; 3:2.

54. Redig PT, Tully TN, Ritchie BW, Roy AF, Baudena MA, Chang GJ. Effect of West Nile virus DNA-plasmid vaccination on response to live virus challenge in red-tailed hawks (Buteo jamaicensis). Am J Vet Res. 2011; 72:1065–1070.

55. Chang GJ, Davis BS, Stringfield C, Lutz C. Prospective immunization of the endangered California condors (Gymnogyps californianus) protects this species from lethal West Nile virus infection. Vaccine. 2007; 25:2325–2330.

56. Quijano-Hernandez IA, Bolio-Gonzalez ME, Rodriguez-Buenfil JC, Ramirez-Sierra MJ, Dumonteil E. Therapeutic DNA vaccine against Trypanosoma cruzi infection in dogs. Ann N Y Acad Sci. 2008; 1149:343–346.
57. Quirk EK, Brown EL, Leavitt RY, et al. Safety profile of the Merck human immunodeficiency virus-1 clade B gag DNA plasmid vaccine with and without adjuvants. Open Forum Infect Dis. 2014 Apr 18 [Epub] http://dx.doi.org/10.1093/ofid/ofu016/.

58. Verdier F, Burnett R, Michelet-Habchi C, Moretto P, Fievet-Groyne F, Sauzeat E. Aluminium assay and evaluation of the local reaction at several time points after intramuscular administration of aluminium containing vaccines in the cynomolgus monkey. Vaccine. 2005; 23:1359–1367.

59. Flarend RE, Hem SL, White JL, et al. In vivo absorption of aluminium-containing vaccine adjuvants using 26Al. Vaccine. 1997; 15:1314–1318.

60. Reyes L, Hartikka J, Bozoukova V, et al. Vaxfectin enhances antigen specific antibody titers and maintains Th1 type immune responses to plasmid DNA immunization. Vaccine. 2001; 19:3778–3786.

61. Vilalta A, Shlapobersky M, Wei Q, Planchon R, Rolland A, Sullivan S. Analysis of biomarkers after intramuscular injection of Vaxfectin-formulated hCMV gB plasmid DNA. Vaccine. 2009; 27:7409–7417.

62. Smith LR, Wodal W, Crowe BA, et al. Preclinical evaluation of Vaxfectin-adjuvanted Vero cell-derived seasonal split and pandemic whole virus influenza vaccines. Hum Vaccin Immunother. 2013; 9:1333–1345.

63. Raviprakash K, Luke T, Doukas J, et al. A dengue DNA vaccine formulated with Vaxfectin(R) is well tolerated, and elicits strong neutralizing antibody responses to all four dengue serotypes in New Zealand white rabbits. Hum Vaccin Immunother. 2012; 8:1764–1768.

64. Veselenak RL, Shlapobersky M, Pyles RB, Wei Q, Sullivan SM, Bourne N. A Vaxfectin((R))-adjuvanted HSV-2 plasmid DNA vaccine is effective for prophylactic and therapeutic use in the guinea pig model of genital herpes. Vaccine. 2012; 30:7046–7051.

65. Sullivan SM, Doukas J, Hartikka J, Smith L, Rolland A. Vaxfectin: a versatile adjuvant for plasmid DNA- and protein-based vaccines. Expert Opin Drug Deliv. 2010; 7:1433–1446.

66. Lin WH, Vilalta A, Adams RJ, Rolland A, Sullivan SM, Griffin DE. Vaxfectin adjuvant improves antibody responses of juvenile rhesus macaques to a DNA vaccine encoding the measles virus hemagglutinin and fusion proteins. J Virol. 2013; 87:6560–6568.

67. Smith LR, Wloch MK, Ye M, et al. Phase 1 clinical trials of the safety and immunogenicity of adjuvanted plasmid DNA vaccines encoding influenza A virus H5 hemagglutinin. Vaccine. 2010; 28:2565–2572.

68. Chiang YW, Jennen CM, Holt TM, et al. Demonstration of efficacy of a West Nile virus DNA vaccine in foals. In : Proceedings of the 51st Annual Convention of the American Association of Equine Practitioners; 2005 Dec 3-7; Seattle, WA. Lexington: American Association of Equine Practitioners;2005. p. 183–190.
69. Trumpfheller C, Caskey M, Nchinda G, et al. The microbial mimic poly IC induces durable and protective CD4+ T cell immunity together with a dendritic cell targeted vaccine. Proc Natl Acad Sci U S A. 2008; 105:2574–2579.

70. Tenbusch M, Ignatius R, Nchinda G, et al. Immunogenicity of DNA vaccines encoding simian immunodeficiency virus antigen targeted to dendritic cells in rhesus macaques. PLoS One. 2012; 7:e39038.

71. Flingai S, Czerwonko M, Goodman J, Kudchodkar SB, Muthumani K, Weiner DB. Synthetic DNA vaccines: improved vaccine potency by electroporation and co-delivered genetic adjuvants. Front Immunol. 2013; 4:354.

72. Takeshita F, Tanaka T, Matsuda T, et al. Toll-like receptor adaptor molecules enhance DNA-raised adaptive immune responses against influenza and tumors through activation of innate immunity. J Virol. 2006; 80:6218–6224.

73. Ullas PT, Desai A, Madhusudana SN. Immunogenicity and efficacy of a plasmid DNA rabies vaccine incorporating Myd88 as a genetic adjuvant. Clin Exp Vaccine Res. 2014; 3:202–211.

74. Liniger M, Summerfield A, Ruggli N. MDA5 can be exploited as efficacious genetic adjuvant for DNA vaccination against lethal H5N1 influenza virus infection in chickens. PLoS One. 2012; 7:e49952.

75. Luo M, Qu X, Pan R, et al. The virus-induced signaling adaptor molecule enhances DNA-raised immune protection against H5N1 influenza virus infection in mice. Vaccine. 2011; 29:2561–2567.

76. Luke JM, Simon GG, Soderholm J, et al. Coexpressed RIG-I agonist enhances humoral immune response to influenza virus DNA vaccine. J Virol. 2011; 85:1370–1383.

77. Dunn MD, Rossi SL, Carter DM, et al. Enhancement of anti-DIII antibodies by the C3d derivative P28 results in lower viral titers and augments protection in mice. Virol J. 2010; 7:95.

78. Capitani M, Saade F, Havas KM, et al. Plasmids encoding protein aggregation domains act as molecular adjuvants for DNA vaccines. Curr Gene Ther. 2014; 14:161–169.

79. Sardesai NY, Weiner DB. Electroporation delivery of DNA vaccines: prospects for success. Curr Opin Immunol. 2011; 23:421–429.

80. Cristillo AD, Weiss D, Hudacik L, et al. Persistent antibody and T cell responses induced by HIV-1 DNA vaccine delivered by electroporation. Biochem Biophys Res Commun. 2008; 366:29–35.

81. Jalah R, Kulkarni V, Patel V, et al. DNA and protein co-immunization improves the magnitude and longevity of humoral immune responses in macaques. PLoS One. 2014; 9:e91550.

82. Hammer SM, Sobieszczyk ME, Janes H, et al. Efficacy trial of a DNA/rAd5 HIV-1 preventive vaccine. N Engl J Med. 2013; 369:2083–2092.

83. Buchbinder SP, Mehrotra DV, Duerr A, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008; 372:1881–1893.

84. Ondondo BO. The influence of delivery vectors on HIV vaccine efficacy. Front Microbiol. 2014; 5:439.

85. Uberla K. Developing an HIV vaccine: the role of efficacy studies in nonhuman primates. PLoS Med. 2005; 2:e119.

86. Johnson TR, Rangel D, Graham BS, Brough DE, Gall JG. Genetic vaccine for respiratory syncytial virus provides protection without disease potentiation. Mol Ther. 2014; 22:196–205.

87. Vasan S, Hurley A, Schlesinger SJ, et al. In vivo electroporation enhances the immunogenicity of an HIV-1 DNA vaccine candidate in healthy volunteers. PLoS One. 2011; 6:e19252.
88. Kopycinski J, Cheeseman H, Ashraf A, et al. A DNA-based candidate HIV vaccine delivered via in vivo electroporation induces CD4 responses toward the alpha4beta7-binding V2 loop of HIV gp120 in healthy volunteers. Clin Vaccine Immunol. 2012; 19:1557–1559.

89. Haynes BF, Gilbert PB, McElrath MJ, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med. 2012; 366:1275–1286.

90. Hartikka J, Geall A, Bozoukova V, et al. Physical characterization and in vivo evaluation of poloxamer-based DNA vaccine formulations. J Gene Med. 2008; 10:770–782.

91. Kharfan-Dabaja MA, Boeckh M, Wilck MB, et al. Reanalysis of TransVax immunogenicity. Lancet Infect Dis. 2013; 13:18.

93. Sandstrom E, Nilsson C, Hejdeman B, et al. Broad immunogenicity of a multigene, multiclade HIV-1 DNA vaccine boosted with heterologous HIV-1 recombinant modified vaccinia virus Ankara. J Infect Dis. 2008; 198:1482–1490.

94. Gudmundsdotter L, Nilsson C, Brave A, et al. Recombinant modified vaccinia Ankara (MVA) effectively boosts DNA-primed HIV-specific immune responses in humans despite pre-existing vaccinia immunity. Vaccine. 2009; 27:4468–4474.

95. Bakari M, Aboud S, Nilsson C, et al. Broad and potent immune responses to a low dose intradermal HIV-1 DNA boosted with HIV-1 recombinant MVA among healthy adults in Tanzania. Vaccine. 2011; 29:8417–8428.

96. Hel Z, Tsai WP, Thornton A, et al. Potentiation of simian immunodeficiency virus (SIV)-specific CD4(+) and CD8(+) T cell responses by a DNA-SIV and NYVAC-SIV prime/boost regimen. J Immunol. 2001; 167:7180–7191.

97. Kent SJ, Zhao A, Best SJ, Chandler JD, Boyle DB, Ramshaw IA. Enhanced T-cell immunogenicity and protective efficacy of a human immunodeficiency virus type 1 vaccine regimen consisting of consecutive priming with DNA and boosting with recombinant fowlpox virus. J Virol. 1998; 72:10180–10188.

98. Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009; 361:2209–2220.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download