Abstract
Purpose
Myeloid differentiation factor 88 (Myd88), a ubiquitous Toll-like receptor adaptor molecule, has been reported to play important roles in B cell responses to infections and vaccination. The present study evaluated the effects of genetic adjuvanting with Myd88 on the immune responses to a plasmid DNA rabies vaccine.
Materials and Methods
Plasmids encoding rabies glycoprotein alone (pIRES-Rgp) or a fragment of Myd88 gene in addition (pIRES-Rgp-Myd) were constructed and administered intramuscularly or intrademally in Swiss albino mice (on days 0, 7, and 21). Rabies virus neutralizing antibody (RVNA) titres were estimated in the mice sera on days 14 and 28 by rapid fluorescent focus inhibition test. The protective efficacy of the constructs was evaluated by an intracerebral challenge with challenge virus standard virus on day 35.
Results
Co-expression of Myd88 increased RVNA responses to pIRES-Rgp by 3- and 2-folds, following intramuscular and intradermal immunization, respectively. pIRES-Rgp protected 80% of the mice following intramuscular and intradermal immunizations, while pIRES-Rgp-Myd afforded 100% protection following similar administrations.
Human and animal mortality from rabies remains a major burden in many countries around the world. Global estimates reveal about 50,000 human deaths from rabies every year, and higher mortality among the livestock [1]. Diverse species of warm-blooded animals transmit the causative agent, a single stranded RNA virus of the genus Lyssavirus of the viral family Rhabdoviridae. Natural exposure to rabies virus through animal bites is very common in the endemic countries of Asia and Africa where companion animals, mainly dogs, are the main vectors of viral transmission to humans [2]. Rabies is 100% fatal but preventable by timely administration of effective pre- or post-exposure vaccination.
At present, mainly cell-culture derived vaccines are used across the world to provide immunity against rabies. However, these expensive, temperature-sensitive biologicals are often not available in many endemic areas. Successful immunization of about 70% of susceptible canine population could prevent rabies transmission among canines [3], but this faces technical and logistic challenges [4]. Canines require annual rabies vaccination from 3 months of age, and often do not develop adequate protective immunity due to poor immunogenicity of the vaccines used, or co-existent malnutrition and diseases. Huge numbers and rapid turnover of free-roaming canines and difficulties in retrieving them for booster vaccinations introduce additional layers of complexity into canine vaccination programmes in the developing countries. Clearly, potent, inexpensive vaccines and efficient mass vaccination strategies are needs of the hour in canine rabies control in the rabies-endemic countries.
Plasmid-based vaccination has been explored as an alternative to cell culture-based rabies vaccines for animal prophylaxis. A eukaryotic expression vector encoding full-length glycoprotein gene of rabies virus represents the simplest design of an anti-rabies vaccine, capable of generating protective neutralizing antibodies upon in vivo delivery. Such constructs have been shown to mediate efficient prophylaxis in small animal models, but are observed to be poorly immunogenic in larger animals ([5], and references therein). In general, systemic lability, poor cellular uptake and low immunogenicity remain major hurdles limiting the utility of plasmids for in vivo applications [6]. Attempts to improve plasmid-based vaccination are currently focused on improved vector design, gene modifications and the use of efficient delivery vehicles and molecular adjuvants [7,8].
Delivery approaches employing gene gun, electroporation, cationic lipids and microparticles, and nanopolymers have been evaluated in improving plasmid-raised immune responses [9,10,11,12,13,14,15]. Molecular adjuvants in the form of gene fragments coding immunomodulatory molecules have also been employed in plasmid vaccination to enhance its immunogenicity and efficacy [16]. Such attempts have generally employed co-administration of discrete plasmids encoding the immunogenic gene and the adjuvant, or constructs designed as fusions of the two. These molecules could be advantageous in achieving site-specific adjuvanting, and limiting adjuvant toxicity [17]. A variety of cytokine, chemokine, pro-apoptotic and other genes have been reported to be effective adjuvants for plasmid-based vaccines [18,19,20,21,22,23,24].
Toll-like receptors (TLRs) are a group of evolutionarily conserved pattern recognition receptors expressed on a wide variety of immune and non-immune cells, that sense specific pathogenic ligands and initiate inflammatory and immune signaling cascades [25]. Their ligands and signaling intermediaries hold considerable promise as immunomodulatory agents [26,27,28].
TLR ligands need to be present extracellularly to bind their cognate receptors, a requirement which increases the risk for their non-specific interactions, systemic toxicity and other adverse events. TLR adaptor molecules, however, act within the cell, limiting the possible toxicity, and quickly achieve threshold levels and faster kinetics. Myeloid differentiation factor 88 (MyD88) is an adaptor molecule essential in signaling through all TLRs except TLR3, and also has roles in signaling through interleukin (IL)-1R1, IL-18R1 and interferon-γ receptor 1 pathways [17]. Studies have reported critical roles for signaling through TLR and MyD88 pathways in the generation of vaccine-generated humoral immunity [27,29,30]. Takeshita et al. [30] reported the enhancement of immunogenicity and protective efficacy of a plasmid-based influenza vaccine, upon the use of Myd88 as a genetic adjuvant.
TLR adaptor molecules have not been investigated previously for their adjuvanting potential in plasmid vaccines against rabies. In the present work, we evaluated Myd88 as a genetic adjuvant in a candidate plasmid rabies vaccine, and report that its effects on the immunogenicity and protective efficacy of the vaccine in Swiss albino mice.
Baby Hamster Kidney (BHK-21) (ATCC CCL-10) was obtained from National Centre for Cell Sciences, Pune, India and maintained at 37℃ under 5% CO2 in Eagle's minimum essential medium supplemented with 10% fetal bovine serum (European Grade FBS, Biological Industries, Beit Haemek, Israel) and antibiotics (100 U/mL of penicillin and 100 µg/mL of streptomycin) (Sigma Aldrich, St. Louis, MO, USA).
The bicistronic eukaryotic expression vector pIRES was provided by Dr. Praveen K. Gupta (Indian Veterinary Research institute, Izatnagar, Uttar Pradesh, India). pFLAG-CMV4-hMyd88, containing an 891 bp fragment of human myeloid differentiation factor primary response gene was a kind gift from Dr. Fumihiko Takeshita, Yokohama City University School of Medicine, Japan. The development of pIRES-Rgp encoding the glycoprotein gene of rabies virus has been reported by us earlier [8]. Large-scale, endotoxin-free plasmid preparations were made using a commercial kit (EndoFree Plasmid Purification Giga Kit, Qiagen, Hilden, Germany).
Polymerase chain reaction (PCR) reagents [Pfu DNA polymerase (Cat. No. EP0571), dNTP mix (Cat. No. R1121)], T4 DNA ligase (Cat. No. EL0014), TurboFect in vitro Transfection Reagent (Cat. No. R0531), Lambda DNA/EcoRI+HindIII marker (Cat. No. SM0193), GeneRuler 100 bp DNA ladder (Cat. No. SM0241) and 6× DNA loading dye (Cat. No. R0611) were purchased from Thermo Scientific (Waltham, MA, USA). The restricton enzymes NheI (Cat. No. R0131), MluI (Cat. No. R0198S), XbaI (Cat. No. R0145S), and SalI (Cat. No. R0138S) and Quick-Load 1 kb DNA ladder (Cat. No. N0486L) were procured from New England Biolabs (Ipswich, MA, USA). Primers to amplify glycoprotein and Myd88 gene fragment were designed from sequences available at NCBI and the plasmid sequence, respectively, using PrimeGen Software (V2 version), and were synthesized at Eurofins Genomics India Pvt. Ltd. (Bangalore, India).
A murine monoclonal antibody against rabies virus glycoprotein, produced as part of an earlier project, was used in indirect immunofluorescent staining procedure for glycoprotein expression. Myd88 expression was probed with a polyclonal rabbit MyD88 antibody (Cat. No. 3699, Cell Signaling Technology, Danveres, MA, USA). The secondary antibody conjugates (goat anti-mouse IgG-FITC [Cat. No. 621120380011730], goat anti-rabbit IgG-TRITC [Cat. No. 621150280011730]) were purchased from Merck Biosciences (Mumbai, India). An in-house anti-rabies serum, calibrated against the Second International Reference Serum (obtained from the National Institute of Biological Standards, Hertfordshire, UK) was employed as a reference serum in the rapid fluorescent focus inhibition test (RFFIT).
A standard laboratory 'fixed' strain (Challenge Virus Standard, CVS-11) of rabies virus, obtained as a freeze-dried mouse brain preparation from the Central Research Institute, Kasauli, India, and maintained by passage in BHK-21 cells and suckling mice, was employed in the in vitro and animal experiments. A commercial rabies vaccine Rabipur (PCEC vaccine, B.No. 1894, Chiron Behring Vaccines Pvt. Ltd., Ankleshwar, India) having a potency ≥2.5 IU/mL was used as a control immunogen.
The plan for animal experiments was approved by the Institutional Animal Ethics Committee, NIMHANS (AEC/35/193(B)/N.V. dated 27.04.2009). Swiss albino mice (4-6 weeks of age and of either sex) were used for the in vivo studies. Care of the animals and the experimental procedures were performed under the guidelines of the Institutional Animal Ethics Committee.
Fig. 1 shows a schematic representation of the proposed plasmid vaccine constructs.
The full-length glycoprotein gene (G) was PCR amplified from pBacPak-GRC9 vector using the primers GFor (ATTAGCTAGCATGGTTCCTCAGGCTCTCC) and GRev (ATGTAGAATTCTCACAGTCTGGTCTGAC) bearing NheI and MluI sites, respectively, and subcloned between these restriction sites in the multiple cloning site-A of pIRES vector, as reported earlier [8]. The construct was named pIRES-Rgp.
An 891 bp fragment of the human Myd88 gene was similarly subcloned into the multiple cloning site-B of pIRES-Rgp. Briefly, the gene fragment was PCR amplified using MydFor (ATTATCTACAATGGCTGCAGGAGGTCCC) and MydRev primers (TAAAGTCGACTCAGGGCAGGGACAAGGC) (bearing XbaI and SalI sites, respectively), using pFLAG-CMV4-hMyd88 as template. The reaction was set up in a volume of 50 µL, employing 10 pmol each of MydFor and MydRev, 2.5 units of Pfu DNA polymerase and 250 ng of the template. The amplification conditions were as follows: denaturation at 95℃ for 1 minute, followed by 35 cycles of 95℃ for 30 seconds, 68℃ for 30 seconds and 72℃ for 45 seconds, and final extension at 72℃ for 3 minutes. The amplicon was gel-purified using GenElute gel extraction kit (Sigma Aldrich) and quantitated. Sequential restriction digestion of pIRES vector and the amplicon was performed with XbaI and SalI enzymes. The digests were again gel-purified, and a ligation reaction was set up with a vector:insert ratio of 1:3 using T4 DNA ligase (Thermo Scientific), in a volume of 20 µL. After overnight incubation at 16℃, 10 µL of the reaction mixture was used to transform competent Escherichia coli DH5α cells by a standard heat-shock procedure. Transformants were subsequently identified on a selective Luria Bertani (LB)-ampicillin agar plate, and the identity of the recombinants was verified by colony PCR using MydFor and MydRev primers. Glycerol stocks of the recombinants were made and stored at -80℃ till further use.
The identities of the recombinant constructs were verified by restriction enzyme analysis and by nucleotide sequencing (data not shown).
Expression of genes encoded in pIRES-Rgp and pIRES-Rgp-Myd was evaluated by indirect immunofluorescent staining of cells transiently transfected with each.
The evaluation of G expression from pIRES-Rgp has been reported earlier [8].
To evaluate expression of G and Myd88 genes from pIRES-Rgp-Myd, transient transfection experiment was set up in BHK-21 cells as before, using 2 µg of the plasmid. Fourty-eight hours post-transfection, the cells were acetone-fixed and subjected to indirect immunofluorescent staining for each protein. Staining for glycoprotein expression was performed as done previously. For verifying MyD88 expression, the cells were probed with a rabbit anti-MyD88 polyclonal antibody (1:100 diluted in phosphate buffered saline [PBS] pH 7.4), followed by goat anti-rabbit IgG-TRITC conjugate (1:100 diluted in PBS pH 7.4). The coverslips were examined under an inverted fluorescence microscope (TS120, Nikon, Tokyo, Japan) and images obtained with the help of a digital camera.
Glycerol stocks of the plasmid constructs were thawed and streaked on to LB ampicillin agar plates. After overnight incubation at 37℃, a single colony was transferred with a sterile inoculating loop into 5 mL of LB ampicillin broth and incubated at 37℃ for 6-8 hours. The starter culture was subsequently inoculated into 50 mL of fresh LB ampicillin broth and grown at 37℃ with vigorous shaking (500 rpm) overnight, which was further inoculated into 2.5 L of fresh LB ampicillin broth and incubated under similar conditions. After 16 hours of growth, the bacteria were pelleted by centrifugation at 5,000 rpm at 4℃. The plasmid identity was once again verified by restriction enzyme analysis of a plasmid miniprep made from 1.5 mL of the overnight culture. Large-scale purification of each plasmid was then performed from the bacterial pellets using a commercial kit (EndoFree Plasmid Purification Giga Kit, Qiagen), as per manufacturer instructions. The quality and concentration of the plasmid preparations were evaluated by spectrophotometry and agarose electrophoresis, and the preparations were stored in aliquots at -20℃ till further use.
Groups (n=10 each) of 6-8-week-old Swiss albino mice of either sex were employed for the experiments. Ninety micrograms of each plasmid, contained in 100 µL or 50 µL of sterile normal saline, was inoculated intramuscularly or intradermally, respectively, into each animal. The intramuscular immunizations were administered into quadriceps muscle on the left leg, and the intradermal inoculations were done at a point about 1 cm below the base of the tail. The animals received one dose of the immunogen on days 0, 7, and 21. Control groups were inoculated with a cell culture rabies vaccine (Rabipur), sterile normal saline, or the empty pIRES vector.
Blood samples were collected from the immunized mice by retro-orbital venous plexus puncture under halothane anesthesia, 14 and 28 days after the first immunization. The samples were allowed to clot, and sera were separated by centrifugation at 6,000 rpm for 5 minutes. Sera were heat-inactivated in a water bath at 56℃ for 30 minutes, and stored at -20℃ till tested.
Rabies virus neutralizing antibody (RVNA) titres in the sera were assayed by a standard RFFIT, with some modifications [31].
The mice were subjected to an intracerebral challenge with 50 LD50 of CVS-11 strain of rabies virus, on day 35. The animals were observed for a period of 28 days for rabies-specific symptoms. At the end of the observation period, the percent survival in each group was recorded. The animals showing symptoms of rabies were euthanized, and infection confirmed by direct fluorescent antibody testing on impression smears prepared from extracted brains.
The statistical analyses were done using GraphPad PRISM software version 5.00 (GraphPad Software, San Diego, CA, USA). Comparison of antibody titres in the different groups was performed with non-parametric ANOVA test, followed by post-hoc analysis by Tukey's multiple comparison test. p<0.05 were considered to be statistically significant.
The development and evaluation of the construct pIRES-Rgp encoding the full-length rabies glycoprotein gene has been reported earlier [8].
The Myd88 fragment (891 bp) was successfully amplified by PCR and cloned into the MCS-B of pIRES-Rgp, between the restriction sites XbaI and SalI, yielding the construct pIRES-Rgp-Myd. Presence of G and Myd88 gene inserts in the construct was checked by PCR using gene-specific primers (Figs. 2, 3), and further verified by restriction enzyme analysis and nucleotide sequencing (data not shown).
BHK-21 cells transiently transfected with pIRES-Rgp revealed expression of the glycoprotein upon indirect immunofluorescent staining using a glycoprotein-specific monoclonal antibody, as reported earlier [8].
Indirect immunofluorescent staining using specific antibodies revealed the expression of both G and MyD88 proteins in BHK-21 cells transfected with pIRES-Rgp-Myd. The transfected cells showed typical apple-green, membrane and cytoplasmic fluorescence, indicative of glycoprotein expression. Orange-red TRITC fluorescence, indicating MyD88 expression, was observed on the cell membranes and in the cytoplasm, in the same fields, indicating probable co-localization of both the proteins (Fig. 4).
In order to prepare the milligram amounts of plasmids required for immunization experiments, we used a commercial large-scale, endotoxin-free plasmid purification kit, as per manufacturer instructions.
We collected sera from the mice on days 14 and 28 post-immunization and estimated their RVNA titres by a RFFIT. The results from a single representative experiment are summarized in Table 1.
As seen from Table 1, RVNA titres>0.5 IU/mL, indicative of sero-conversion for rabies, developed by day 14 in all groups of mice immunized with pIRES-Rgp or pIRES-Rgp-Myd. We observed the highest RVNA titres in the group that received two doses of Rabipur (8 IU/mL), followed by the group immunized with pIRES-Rgp-Myd (3.2 IU/mL). Notably, pIRES-Rgp-Myd induced ~3.2 fold higher levels of antibodies than pIRES-Rgp (p<0.05) at this time point.
The RVNA titres increased further in the groups, after the booster dose on day 21 (Table 1). On day 28, the highest titres of neutralizing antibodies were still seen in the Rabipur group (14.4 IU/mL). Mice immunized with pIRES-Rgp-Myd showed a mean titre of 11.2 IU/mL. The mean titre observed in groups immunized with pIRES-Rgp at this point was 3.6 IU/mL. The RVNA titres increased >3-fold in these groups, following the booster dose on day 21. The RVNA titres following intramuscular immunization with pIRES-Rgp-Myd were 3.1-fold higher than those induced by pIRES-Rgp at this point (p<0.05).
Among the intradermally immunized groups, the highest RVNA titres at day 14 were detected in the group immunized with Rabipur (8 IU/mL) (Table 1). pIRES-Rgp-Myd and pIRES-Rgp induced titres of 3.0 and 0.96 IU/mL respectively, at this time point. We observed a 2.5-fold increase in RVNA titre following a booster with pIRES-Rgp-Myd, whereas the pIRES-Rgp booster increased it by 3.6-fold.
We evaluated the protective efficacy of the plasmid constructs by an intracerebral challenge study with 50 LD50 of CVS-11 strain of rabies virus, on day 35. The inoculated animals were observed for a period of 21 days for clinical symptoms of rabies, and the percentage survival was recorded in each group. Mice inoculated with the empty vector pIRES and normal saline developed clinical symptoms of rabies by day 6 and were euthanized. We observed a survival rate of 80% in the group immunized intramuscularly with pIRES-Rgp (Fig. 5). Notably, pIRES-Rgp-Myd protected 100% of the animals following intramuscular and intradermal immunization. pIRES-Rgp, however, protected only 80% of the mice following immunization via either route. All the animals immunized with Rabipur survived the challenge.
Despite successful demonstrations of efficacy of plasmid DNA-based vaccination strategy for rabies in a number of animal models, the technology is still constrained by poor immunogenicity observed in larger animals and the delay in onset of antibody responses. A number of physical, chemical and biological methods have been developed and evaluated for enhancing DNA vaccine efficacy, of which, in vivo electroporation and gene gun delivery have been found to yield the highest efficacy [32].
Neutralizing antibodies directed against surface proteins of the pathogen represent crucial correlates of protective immunity in a number of infections, including rabies. Generation and proliferation of antibody-secreting plasma B-cells and the maintenance of antigen-specific memory B-cells underlie efficient and long-lasting humoral immune responses. The signaling molecules of the TLR pathway offer opportunities to influence these phenomena, in view of their ubiquitous expression, and ability to modulate multiple signaling pathways [33].
Experimental evidence abounds on the ability of Myd88 to influence immune and inflammatory signaling through multiple pathways, and protective immunity against many pathogens [26,28,34,35,36,37,38,39]. In view of reports suggesting the important roles played by Myd88 signaling in the generation of humoral immune responses, we explored the potential utility of Myd88 as a genetic adjuvant in plasmid vaccination against rabies.
Our study revealed that co-expression of Myd88 in a plasmid rabies vaccine enhanced the RVNA titres in Swiss albino mice upon intramuscular and intradermal immunization (Table 1). Notably, the mean RVNA titre at day 28 was 3-fold higher in the group immunized intramuscularly with pIRES-Rgp-Myd than in the group immunized with pIRES-Rgp (p<0.05). This is in agreement with Takeshita et al. [30] who reported enhancement in antibody titres upon Myd88 adjuvanting in a plasmid vaccine against influenza. pIRES-Rgp-Myd increased the RVNA titres > 2-fold upon intradermal immunization, compared to pIRES-Rgp (p<0.05).
We observed comparable antibody titres in groups receiving pIRES-Rgp intramuscularly and intradermally. This observation is intriguing, as it is known that intradermal immunization generally induces better immune responses than the intramuscular immunization.
In a previous study, Pinto et al. [23] studied the effect of co-administration of plasmids encoding chemokine and cytokine genes (RANTES, MCP-1, MIP-1-β, and TRANCE) in plasmid vaccines against rabies, and observed a modest adjuvant effect to a secreted and truncated form of the protein. The authors commented that genetic adjuvants may be unable to influence early events of immune response, due to temporal differences in expression of the immunogen and the adjuvant genes. The present study, in contrast, evaluated immune responses to plasmids co-expressing viral glycoprotein and Myd88 genes. Co-expression of both the genes was appreciable in fluorescently-stained transfected cells, and it would seem likely that MyD88 expression could have influenced activation and maturation of antigen presenting cells at the site of inoculation.
An early onset and a high initial titre of RVNA would be ideal in the context of rabies vaccination. Whereas the former holds critical importance in post-exposure prophylaxis, the decline of antibody responses to non-protective levels can be delayed by high initial titres of RVNA. This would be beneficial in maintaining protective immunity over long periods in immunized animals, especially in canines. Findings from the study appear to suggest the ability of Myd88 adjuvanting in influencing these two relevant attributes of protective immunity against rabies.
We evaluated the protective efficacy of the vaccine formulations by a viral challenge study performed on the immunized animals on day 35. pIRES-Rgp protected 80% of the immunized animals against the challenge, following intramuscular and intradermal routes of administration. Notably, 100% of the animals immunized with pIRES-Rgp-Myd (in either route) survived the challenge (Fig. 5).
Kaur et al. [40] reported variation in protective efficacy against a viral challenge despite similar magnitude of immune response to different plasmid vaccine constructs, suggesting the probable contribution of cellular immune factors in protective immunity against rabies. Findings from the present study seem to support this, as complete protection against the lethal virus challenge was not observed in the groups immunized with pIRES-Rgp. We propose that the enhanced RVNA responses and protective efficacy following immunization with pIRES-Rgp-Myd might have resulted from stimulation of the cellular immune responses.
In conclusion, the study reveals that genetic adjuvanting with Myd88 considerably enhances the protective immune responses to plasmid-based rabies vaccines. Plasmid vaccines encoding Myd88 in addition to the protective immunogen might represent efficacious vaccine candidates for generation of long-lasting protective immunity against rabies and other infectious diseases, especially for immunization of animals, and needs to be evaluated further in field conditions.
Figures and Tables
 | Fig. 2Gel image showing polymerase chain reaction amplification of rabies virus glycoprotein gene. Lane 1, lambda DNA/EcoRI+HindIII marker; lane 2, ~1.6 kb band indicative of full-length glycoprotein gene. |
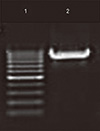 | Fig. 3Gel image showing polymerase chain reaction amplification of Myd88 gene fragment. Lane 1, GeneRuler 100 bp DNA ladder; lane 2, 891 bp band indicative of Myd88 gene fragment. |
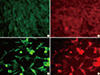 | Fig. 4Images of BHK-21 cells fluorescently stained for expression of G and MyD88. Cells were grown in 24-well plates and mock-transfected (A, B) or transfected with a liposomal complex of pIRES-Rgp-Myd (C, D). Forty-eight hours later, the cells were stained sequentially with a murine anti-G antibody and a rabbit polyclonal anti-Myd88 antibody, followed by species-specific secondary IgG conjugated with FITC or TRITC, respectively. Note the lack of specific fluorescence in mock-transfected cells, and the apple-green fluorescence (indicative of expression of G) and orange-red fluorescence (indicative of MyD88 expression) in pIRES-Rgp-Myd transfected cells, following staining (images at a total magnification of ×400). |
References
1. Knobel DL, Cleaveland S, Coleman PG, et al. Re-evaluating the burden of rabies in Africa and Asia. Bull World Health Organ. 2005; 83:360–368.
3. World Health Organization. Guidelines for dog rabies control. VH/83.43 Rev. 1. Geneva: WHO;1986.
4. Bahloul C, Taieb D, Diouani MF, et al. Field trials of a very potent rabies DNA vaccine which induced long lasting virus neutralizing antibodies and protection in dogs in experimental conditions. Vaccine. 2006; 24:1063–1072.

5. Ullas PT, Desai A, Madhusudana SN. Rabies DNA vaccines: current status and future. World J Vaccines. 2012; 2:36–45.

6. Barry ME, Pinto-Gonzalez D, Orson FM, McKenzie GJ, Petry GR, Barry MA. Role of endogenous endonucleases and tissue site in transfection and CpG-mediated immune activation after naked DNA injection. Hum Gene Ther. 1999; 10:2461–2480.

7. Ulmer JB, Wahren B, Liu MA. Gene-based vaccines: recent technical and clinical advances. Trends Mol Med. 2006; 12:216–222.

8. Ullas PT, Madhusudana SN, Desai A, et al. Enhancement of immunogenicity and efficacy of a plasmid DNA rabies vaccine by nanoformulation with a fourth-generation amine-terminated poly (ether imine) dendrimer. Int J Nanomedicine. 2014; 9:627–634.
9. Singh M, Briones M, Ott G, O'Hagan D. Cationic microparticles: a potent delivery system for DNA vaccines. Proc Natl Acad Sci U S A. 2000; 97:811–816.

10. Lodmell DL, Parnell MJ, Bailey JR, Ewalt LC, Hanlon CA. Rabies DNA vaccination of non-human primates: post-exposure studies using gene gun methodology that accelerates induction of neutralizing antibody and enhances neutralizing antibody titers. Vaccine. 2002; 20:2221–2228.

11. Margalith M, Vilalta A. Sustained protective rabies neutralizing antibody titers after administration of cationic lipid-formulated pDNA vaccine. Genet Vaccines Ther. 2006; 4:2.
12. Bodles-Brakhop AM, Heller R, Draghia-Akli R. Electroporation for the delivery of DNA-based vaccines and immunotherapeutics: current clinical developments. Mol Ther. 2009; 17:585–592.

13. Kaur M, Saxena A, Rai A, Bhatnagar R. Rabies DNA vaccine encoding lysosome-targeted glycoprotein supplemented with Emulsigen-D confers complete protection in preexposure and postexposure studies in BALB/c mice. FASEB J. 2010; 24:173–183.

14. Xiang SD, Selomulya C, Ho J, Apostolopoulos V, Plebanski M. Delivery of DNA vaccines: an overview on the use of biodegradable polymeric and magnetic nanoparticles. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2010; 2:205–218.

15. Nawwab Al-Deen FM, Selomulya C, Kong YY, et al. Design of magnetic polyplexes taken up efficiently by dendritic cell for enhanced DNA vaccine delivery. Gene Ther. 2014; 21:212–218.

16. Kobiyama K, Jounai N, Aoshi T, et al. Innate immune signaling by, and genetic adjuvants for DNA vaccination. Vaccines. 2013; 1:278–292.

17. Wales J, Andreakos E, Feldmann M, Foxwell B. Targeting intracellular mediators of pattern-recognition receptor signalling to adjuvant vaccination. Biochem Soc Trans. 2007; 35(Pt 6):1501–1503.

18. Xiang Z, Ertl HC. Manipulation of the immune response to a plasmid-encoded viral antigen by coinoculation with plasmids expressing cytokines. Immunity. 1995; 2:129–135.

19. Geissler M, Gesien A, Tokushige K, Wands JR. Enhancement of cellular and humoral immune responses to hepatitis C virus core protein using DNA-based vaccines augmented with cytokine-expressing plasmids. J Immunol. 1997; 158:1231–1237.
20. Tsuji T, Hamajima K, Ishii N, et al. Immunomodulatory effects of a plasmid expressing B7-2 on human immunodeficiency virus-1-specific cell-mediated immunity induced by a plasmid encoding the viral antigen. Eur J Immunol. 1997; 27:782–787.

21. Xiang ZQ, He Z, Wang Y, Ertl HC. The effect of interferon-gamma on genetic immunization. Vaccine. 1997; 15:896–898.
22. Sin JI, Kim JJ, Ugen KE, Ciccarelli RB, Higgins TJ, Weiner DB. Enhancement of protective humoral (Th2) and cell-mediated (Th1) immune responses against herpes simplex virus-2 through co-delivery of granulocyte-macrophage colony-stimulating factor expression cassettes. Eur J Immunol. 1998; 28:3530–3540.

23. Pinto AR, Reyes-Sandoval A, Ertl HC. Chemokines and TRANCE as genetic adjuvants for a DNA vaccine to rabies virus. Cell Immunol. 2003; 224:106–113.

24. Bramson JL, Dayball K, Hall JR, et al. Super-activated interferon-regulatory factors can enhance plasmid immunization. Vaccine. 2003; 21:1363–1370.

25. O'Neill LA, Golenbock D, Bowie AG. The history of Toll-like receptors: redefining innate immunity. Nat Rev Immunol. 2013; 13:453–460.
26. Genestier L, Taillardet M, Mondiere P, Gheit H, Bella C, Defrance T. TLR agonists selectively promote terminal plasma cell differentiation of B cell subsets specialized in thymus-independent responses. J Immunol. 2007; 178:7779–7786.

27. O'Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007; 7:353–364.
28. Rubtsov AV, Swanson CL, Troy S, Strauch P, Pelanda R, Torres RM. TLR agonists promote marginal zone B cell activation and facilitate T-dependent IgM responses. J Immunol. 2008; 180:3882–3888.

30. Takeshita F, Tanaka T, Matsuda T, et al. Toll-like receptor adaptor molecules enhance DNA-raised adaptive immune responses against influenza and tumors through activation of innate immunity. J Virol. 2006; 80:6218–6224.

31. Smith JS, Yager PA, Baer GM. A rapid reproducible test for determining rabies virus-neutralizing antibody. In : Meslin FX, Kaplan MM, Koprowski H, editors. Laboratory techniques in rabies. 4th ed. Geneva: World Health Organization;1996. p. 181–192.
33. Gururajan M, Jacob J, Pulendran B. Toll-like receptor expression and responsiveness of distinct murine splenic and mucosal B-cell subsets. PLoS One. 2007; 2:e863.

34. Ha SA, Tsuji M, Suzuki K, et al. Regulation of B1 cell migration by signals through Toll-like receptors. J Exp Med. 2006; 203:2541–2550.

35. Guay HM, Andreyeva TA, Garcea RL, Welsh RM, Szomolanyi-Tsuda E. MyD88 is required for the formation of long-term humoral immunity to virus infection. J Immunol. 2007; 178:5124–5131.

36. von Bernuth H, Picard C, Jin Z, et al. Pyogenic bacterial infections in humans with MyD88 deficiency. Science. 2008; 321:691–696.

37. Sheahan T, Morrison TE, Funkhouser W, et al. MyD88 is required for protection from lethal infection with a mouse-adapted SARS-CoV. PLoS Pathog. 2008; 4:e1000240.

38. Neves P, Lampropoulou V, Calderon-Gomez E, et al. Signaling via the MyD88 adaptor protein in B cells suppresses protective immunity during Salmonella typhimurium infection. Immunity. 2010; 33:777–790.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download