Abstract
Purpose
FimH (the adhesion fragment of type 1 fimbriae) is implicated in uropathogenic Escherichia coli (UPEC) attachment to epithelial cells through interaction with mannose. Recently, some studies have found that UPEC can thrive intracellularly causing recurrent urinary tract infection (UTI). Almost all vaccines have been designed to induce antibodies against UPEC. Yet, the humoral immune response is not potent enough to overcome neither the primary UTI nor recurrent infections. However, DNA vaccines offer the possibility of inducing cell mediated immune responses and may be a promising preventive tool.
Materials and Methods
In this study, we employed two different open reading frames within mammalian (mam) and wild type (wt) codons of fimH gene. Optimized fragments were cloned in pVAX-1. Expression of the protein in COS-7 was confirmed by western blot analysis after assessing pVAX/fimH(mam) and pVAX/fimH(wt). The constructs were injected to BALB/c mice at plantar surface of feet followed by electroporation.
Results
The mice immunized with both constructs following booster injection with recombinant FimH showed increased interferon-γ and interleukin-12 responses significantly higher than non-immunized ones (p<0.05). The immunized mice were challenged with UPEC and then the number of bacteria recovered from the immunized mice was compared with the non-immunized ones. Decreased colony count in immunized mice along with cytokine responses confirmed the promising immune response by the DNA vaccines developed in this study.
Urinary tract infections (UTIs) are the second most common infectious disease [1]. An estimated 150 million humans develop UTI each year worldwide [2]. Approximately 90% of common UTIs, are caused by uropathogenic Escherichia coli (UPEC) strains. More than 90% of all E. coli isolates from UTI patients express type 1 fimbriae (or mannose-sensitive fimbriae) which is known as a major virulence factor of UPEC. Type 1 pilli of the bacterial surface mediate bacterial attachment to host cells via the adhesion protein FimH located on the tip of the pilus [3].
The function of UPEC type 1 fimbriae is two-fold in bladder 1) induction of apoptosis in superficial cells leading to the loss of superficial urothelial cells (dependent upon the complete FimH portion, while the UTI89 ΔfimH[type1+/FimH-] strain failed to induce apoptosis) [4]. 2) Adhesion of UPEC to urothelial cells, UPEC colonization and establishing intracellular reservoirs. FimH attachment to urothelial cells may result in apoptosis which can be a host defense in response to interactions with uropathogens [5]. In other words, host response may promote invasion/reservoir formation that support intracellular proliferation and recurrent infections.
Innate immune response, the first line defense against bacteria, may not always succeeded against UPEC leading to the transcytosis of bacteria through the mucosal barrier and causing acute UTI. UPEC has developed mechanisms to respond to host recognition and signaling. One of the immune evasion strategies used by certain pathogens is the TcpC-mediated interference of MyD88 signaling, which deteriorates innate immune responses. TcpC of UPEC clinical isolates, which has structural homology to the TIR domain of human Toll-like receptor 1, binds to MyD88 and inhibits cytokine responses [6]. Billips et al. [7] screened for UPEC transposon mutants and recognized a peptidoglycan permease (ampG) and an O-antigen ligase gene (waaL) responsible for the dulled cytokine secretion in response to uropathogenic strains. A similar study showed that rfa-rfb operons and surA genes were essential for lipopolysaccharide (LPS) biosynthesis and outer membrane protein biogenesis, respectively [8]. These findings propose that UPEC employs gene products that alter bacterial surface (especially LPS) to evade immune recognition emphasizing on the potential importance of Toll-like receptor stimulation by fimbriae and other organelles. Upon FimH-mediated internalization, UPEC forms intracellular bacterial community (IBC) and persists as intracellular bacteria. Therefore, after treatment with antibiotics patients usually experience recurrent UTI. Moreover, long-term prophylactic administration of antibiotics in patients with recurrent infections increases possibility of emergence of antibiotic-resistant UPEC strains. For instance trimethoprim-sulfamethoxazole combination that is commonly used to treat UTIs, fails to clear bacteria from bladder and the same pattern of resistance also occurres in ex vivo treatment with gentamycin [9]. Thus, prevention and treatment of recurrent UTIs due to UPEC is desirable from both medical and economic standpoints.
The current data on adaptive immune responses to UPEC are relatively scarce, particularly in the field of cellular immunity. Nevertheless, CD4 and CD8 cells in response to reinfection, infiltrate the bladder and express activation marker (CD69) in the spleen. According to available studies, there is no significant tendency towards Th1- or Th2-mediated UTI immunity. In this aspect, cellular immunity along with humoral immunity are essential to treatment and prevention of recurrent UTI [10].
An efficient vaccine has yet to be designed to protect against UTI infections in humans. The current antigen-containing vaccines have been developed to trigger humoral responses. However, little success has been gained in prevention and treatment of recurrent UTIs. The advent of DNA vaccines and their use in preclinical studies for eliminating viral and non-viral antigens has generated high hopes in the field of vaccinology. In theory, DNA vaccines can generate a wide range of responses in the same way attenuated live viruses can do so, without the requirement for replicating the pathogen [11]. In spite of recent advances in creating several DNA vaccines as a desirable preventive tool, the results in animal models can hardly be generalized to humans [12,13]. Therefore, the low immunogenicity of DNA vaccines caused the researchers to search for new approaches of delivery strategies, codon optimization and different kinds of molecular adjuvants.
Thus, we optimized fimH gene in eukaryotic codon usage to be compatible with eukaryotic expression system. Electoporation delivery system was used to enhance host immune responses. In an independent study [14], we confirmed the expression of current DNA vaccine in vitro; in the present study, we intended to examine the induction of cellular immunity against recurrent UTI in response to DNA vaccine in BALB/c mice.
Plasmids pVAX/fimH(mam) and pVAX/fimH(wt) were constructed in our laboratory as described previously [14]. Briefly, fimH(mam) comprised of mammalian (mam) codon usage sequences of FimH protein and fimH(wt) composed of wild type (wt) codon usage sequences of FimH protein. The fimH(mam) and fimH(wt) were cloned into the vector pVAX1 containing an anti-kanamycin gene (Invitrogen, Carlsbad, CA, USA) to create pVAX/fimH(mam) and pVAX/fimH(wt) constructs. DNA vaccine was prepared using an Endo Free Plasmid Giga-prep Kit (Qiagen, Hilden, Germany).
To study the immune responses to FimH antigen as a UTI vaccine, six- to eight-week-old female BALB/c mice were purchased from Pasteur Institute Laboratory Animal Center (Tehran, Iran) and maintained in the animal facility under standard conditions. All animal experimentations were performed according to the guidelines of the National Institutes of Health (http://oacu.od.nih.gov/training/index.htm). The mice were kept in plastic cages containing water bottles, and maintained on a 12:12 hour light and dark cycle during the study period. Temperature and humidity were adjusted at 23±1℃ and 55±10%, respectively. All mice were fed a standard mouse pellet diet. Five groups of 12 mice were treated as follows: two groups were immunized with either mammalian or wild type DNA vaccines plus recombinant FimH, one group was immmunized with recombinant protein only, and two groups were immunized with either empty vector (pVAX-1) or phosphate buffered saline (PBS) as negative control. For immunization with the DNA vaccine, electroporation (Elelectro square porator, Harvard Apparatus, Holliston, MA, USA) was used as a delivery system. For the electroporation, the BALB/c mice were anaesthetized with a mixture of ketamine and xylazine. Mice were then injected with 5 µg of endotoxin-free pDNA in 50 µL of PBS/animal in the right planetary surface of the feet. Three pulses of 6 V each were delivered to the injection site at a rate of one pulse per second, each lasting for 20 milli per seconds. Mice were injected with the boosters four times at eight weeks intervals via insulin syringe subcutaneously in right planetary surface of feet. In the recombinant protein group, FimH protein was injected subcutaneously along with incomplete Freund adjuvant. Control mice were injected with either pVAX-1 or PBS.
Mice were sacrificed, and spleens were removed. Spleen cells were isolated by grinding the dissected spleens between frosted microscope slides. Erythrocytes were lysed with 0.1 M ammonium chloride. The spleen cells were then washed three times with sterile PBS and then suspended in complete RPMI 1640 (supplemented with 10% heat-inactivated fetal bovine serum) (Gibco, Darmstadt, Germany). Viability was determined by trypan blue exclusion method. Splenocytes were cultured in triplicates (1×106 cells/well) in a 24-well culture plate (Nunc, Copenhagen, Denmark), stimulated with 5 µg/mL of recombinant FimH, and incubated at 37℃ under 5% CO2 and 95% humidity. Supernatants were harvested after 72 hours and the levels of cytokines quantified by enzyme-linked immunosorbent assay (ELISA). Bromo-deoxyuridine (BrdU) assay was used to check for cell proliferation. After incubating the cells for 68 hours, 20 µL/well BrdU labeling solution was added and reincubated for additional 4 hours at 37℃, and then proliferation assay was carried out based on a colorimetric BrdU assay procedure (Roche, Basel, Switzerland). Stimulation index was obtained from mean ratio of the stimulated cells optical density to that of the cells without stimulation.
Levels of interleukin (IL)-4, interferon-γ (IFN-γ), IL-17, and IL-12 were determined using ELISA kits (R&D Systems, Minneapolis, MN, USA) according to the manufacturer's protocol. Briefly, a 96-well microtiter ELISA plate (MaxiSorb, Nunc) was coated with capture antibody of the respective cytokines and incubated overnight at 4℃. Wells were aspirated, washed 3 times, and subsequently blocked with 200 µL of 2% bovin serum albumin for 2 hours at 37℃. After the incubation period, wells were aspirated, washed 3 times, and then incubated with the harvested supernatants for 2 hours at room temperature (RT). The wells were then aspirated, washed 5 times, and incubated with the labeled secondary antibody (anti-mouse IgG-horseradish peroxidase) for 1 hour at RT. Then wells were aspirated, washed 7 times, and incubated with 100 µL of substrate solution for 30 minutes in the dark at RT. The reaction was stopped by adding 50 µL of stop solution (2N H2SO4) to each well. The absorbance was read at 450 nm using a microplate reader (BioRad Laboratories, Hercules, CA, USA) within 30 minutes of stopping the reaction. The concentrations of cytokines in the supernatants were calculated using a linear regression equation obtained from the absorbance values of the standards provided by the manufacturer.
Challenge with the uropathogenic strain, E. coli 35218, was performed by inoculating the organism into the bladder of mice two weeks after the last immunization. To make the bacterial suspension, a 48-hour culture of the E. coli 35218 was harvested, washed, and adjusted to 1×1010 CFU/mL. Mice were anesthetized with ketamine (Ketamine, 75 mg/kg, i.p.; Yuhan, Seoul, Korea) and xylazine (Rompun, 15 mg/kg, i.p.; Bayer Korea Ltd., Seoul, Korea) and inoculated trans-urethrally with 10 µL bacterial suspension containing 1×108 CFU by means of a sterile polyethylene catheter [15]. Two days after inoculation, the bladder of mice were excised and homogenized in 1 mL of sterile PBS by means of pellet mixer (Treff Lab, Degersheim, Switzerland). Serial dilutions of the homogenates were cultured on Luria broth medium, and recovered colonies were counted.
Bladders of mice from the same groups were removed at the same time, fixed with 1% formalin, and evaluated histopathologically. Sections of 5-µm thick were cut from paraffin-embedded tissues and stained with hematoxylin and eosin (H&E).
ELISA was used according to the manufature protocol (Abcam, Cambridge, MA, USA). IgA titers were determined by serially diluting the samples (urine) and reading the absorbance of 0.2 above background at 400 nm after addition the tube-bound enzyme with substrate for 3 hours.
Statistical analysis of the specific interleukin levels and caries scores were performed with SPSS version 20.0.0 (SPSS Inc., IBM Co., Armonk, NY, USA). Differences between experimental and control groups were determined by one-way analysis of variance (ANOVA) and considered significant if p-values were <0.05. The results of challenge with EPEC were analyzed with the Kruskal-Wallis with Dunn's multiple comparison, using Prism software (GraphPad). p<0.05 of all data was considered as significant.
Cell proliferation assay with BrdU showed significantly higher response in the DNA vaccine with recombinant FimH booster (pV-FimH[mam]+boost and pV-FimH[wt]+boost) compared to the backbone control (pV[ctrl]) and PBS control group (p<0.05). Stimulation with FimH protein (pro) also led to significantly higher degree of proliferations compared to the backbone control (pV[ctrl]) as well as PBS control group (p<0.05) (Fig. 1).
The levels of IFN-γ, IL-12, and IL-4 in the spleen cell culture supernatants were measured using a sandwich ELISA assay (R&D System). As Fig. 2A shows, the level of IFN-γ was significantly higher in the DNA vaccine group than in the control groups (p<0.002). It was also found that there is a significant difference between DNA vaccine (pV-FimH[mam]/[wt]+boost) groups and protein-only group in terms of IFN-γ (p<0.02). There were no significant differences between optimized (pV-FimH[mam]) and wild type (pV-FimH[wt]) DNA vaccines. Fig. 2B shows a significant increased level of IL-12 in the test groups compared to the control groups (p<0.001). These results indicate that the Th1 type of immune response was more likely to be involved in the test mice than in the control mice. Fig. 2C shows that the level of IL-4 in the spleen cell culture supernatants was not elevated significantly (p>0.05) in the test groups. Fig. 2D shows the level of IL-17 was significantly higher in the DNA vaccine group than in the control groups (p<0.002) and also indicates that significantly higher amounts of IL-4 was produced in the DNA vaccine (pV-FimH[mam]/[wt]+boost) groups than the one in the protein group (p<0.02).
Five groups of 5 mice were used in the bacterial challenge. As expected, the mice immunized with E. coli 35218 (1×108 CFU) most efficiently decreased the infected bacteria. The bacterial count was noticeably lower among the DNA vaccine groups compared with the control groups (p<0.05). There is no significant difference between protein and control groups in terms of the recovered bacteria post-challenge (p>0.05) (Fig. 3). Also our results revealed that both mammalian and wild type DNA vaccines are equally effective and there is no significant difference between them. To confirm the bacterial colonization in the tissue, the excised bladders were investigated histologically and stained with H&E (Fig. 4). Fig. 4A and B show bacterial spots deposited on the epithelial layers confirming the bacterial adhesion to the mucosal surfaces of bladder while there is no evidence of bacterial attachment in negative control unchallenged mice (Fig. 4C).
IgA secretion in DNA vaccine group (pV-FimH[mam]/[wt]+ boost) and protein group was significantly higher than control groups (p<0.001). There were no significant difference between optimized mammalian (pV-FimH[mam]) and wild type (pV-FimH[wt]) DNA vaccine. These results indicate that increased levels of IgA correspond with the decline of bacterial attachment in the test mice (Fig. 5).
Firstly, evaluation of IFN-γ, IL-12 and IL-4 secretion upon administration of the newly designed UTI DNA vaccine indicated that it induces Th1 type immune responses primarily in the test mice. According to a recent study, IL-17 was evaluated and promoted protective effects in mice [16]. The protective effects of pV-FimH vaccine were accompanied by the involvement of innate and adaptive immune responses, eliciting Th1 and Th17 cell responses, respectively. IL-17 is a cytokine produced by a subtype of T-helper cells and like IFN-γ acts as a potent mediator in delayed-type reactions by increasing chemokine production in various tissues to recruit monocytes and neutrophils to the site of inflammation.
Second, the optimized DNA vaccine with mammalian codon usage could stimulate cellular immune response similar to the DNA vaccine with wild type codon usage. Moreover, in challenge with UPEC we found that both of the DNA vaccines (mam and wt) could provide higher degrees of protection compared to the protein or negative control groups (pV[ctrl] and PBS).
As hypothesized, codon usage optimization could not stimulate higher immune responses than wild type codon usage, but it is important that the optimized codon usage could be used instead of the wild type not only for the higher expression capacity but also for a better compatibility with eukaryotic expression system.
In this study, our hypothesis was based on the UPEC life style in the microenvironment of the urinary tract epithelial cells and that the newly developed DNA vaccine could induce cellular immune responses. Briefly, different stages of UPEC colonization include bacterial attachment, UPEC invasion to epithelial cells and formation of IBC. A major benefit of IBC formation is that it confers protection against antibiotics both in vitro and in vivo [17,18]. Aside from IBC formation and recurrent of the growth cycles a quiescent bacterial stage is developed in the bladder that could be a reservoir for subsequent UTI. Also type 1 pili, with the FimH adhesin at the distal tip, are crucial for UPEC attachment to the bladder epithelium [4]. For these reasons, stimulating the cellular immunity with DNA vaccine containing fimH gene may be a novel idea which is different from other vaccines that trigger antibody production against Dr fimbriae [16], type 1 fimbria [19], FimHt [20], and FimC-H [21,22]. DNA vaccine as a great approach in stimulating both cellular and humoral immune responses is still under investigation to find the most efficient method of optimization. One of the methodologies in developing DNA vaccines is codon usage optimization [22]. Recently, Supek and Smuc [23] found that the expression of green fluorescent protein in Escherichia coli variants was not affected by codon bias, whereas stability of an mRNA secondary structure near the 5' end played a main role. In this study, we used Mus musculus codon usage with 11.9% C+G content higher than wild type gene. Some studies showed that optimization of coding sequences resulted in not only substantial improvement in protein expression by mammalian cells but also induced stronger Th1-like and cytotoxic T-cell immune responses in BALB/c mice [24]. Also other studies showed that codon optimization increases stable-state mRNA levels [25]. Although the expression of optimized fimH gene was higher than wild type gene, we did not observe any significant differences between optimized and wild type fimH gene in terms of cytokine production (data not shown). On the other hand, Uchijima et al. [26] supported that substitution of wild type codon usage with codons frequently found in highly expressed murine genes, generated Tc1 responses. Codon optimization can improve expression of human genes in E. coli: A multi-gene study [27]. Wang et al. [28] found a significant increase in cysC expression in E. coli produced by codon optimization techniques and they improved the protein expression from 10% to 46% based on total protein expression after codon optimization. Our data indicated that DNA vaccination (pVax/fimH+FimH boosting) reduced bladder infection about 103 to 104 fold compared to the control mice. The highest prevention was observed in DNA vaccine groups containing pV-fimH+boost. The DNA vaccine could decrease the colony count in upon challenge with UPEC. Asadi Karam et al. [29] used FimH and FliC protein plus montanide as an adjuvant and reported that their vaccine (FimH+FliC+montanide) reduced bladder infection about 105 fold. It is worth noticing that their vaccine had the advantage of inducing strong and long-lasting humoral and cellular (Th1 ad Th2) immune responses while our vaccination lead to cellular responses only.
Despite of the capacity of humoral responses to reduce bladder infections, this type of immunity is unable to reduce IBCs. Thus, the main advantage of our DNA vaccine, was that it promoted cellular immune responses and reduced intracellular communities and finally reduced the frequency of chronic or recurrent infections caused by IBCs.
Inefficient uptake of the plasmids by cells due to ineffective delivery system is one of the reasons of low immunogenicity of early DNA vaccines. Electroporation is the application of short electrical pulses to deliver DNA vaccines into the tissues resulting in an increase in antigen production [30] and consequently vaccine immunogenicity [31]. Also electroporation augmentes both antigen specific production of IFN-γ and seroconversion [11]. Therefore we used electroporation system with some modifications in this study.
Secretory immunoglobulin A (sIgA) is essential to protecting mucosal surfaces. It is more potent when bound to secretory component (SC) in protecting mice against bacterial infection of the respiratory tract and urine infection. The SC plays an important role in protecting sIgA against proteolytic degradation in the gastrointestinal tract [32] and the oral cavity [33]. It also neutralizes bacterial toxins and inhibits bacterial adhesion by direct nonspecific binding [34]. Our data show the correlation between Ab and bacterial colonization. When mice were immunized with pVax/fimH(mam), the level of IgA antibody was raised and consequently the infection and colonization of the bacteria were decreased.
The data imply that both DNA vaccines (pV-FimH[mam]+boost and pV-FimH[wt]+boost) have the potential to cause Th1 polarization in the BALB/c mice. Since IL-12 selectively promotes the differentiation of Th0 to Th1 cells, the induction of IL-12 by UTI DNA vaccine may regulate T-helper polarization in BALB/c mice. This study can be a preliminary work and the first study of DNA vaccine in recurrent UTI. The data suggested that the DNA vaccines without molecular adjuvant, conjugated genes or improved formulation could not induce effective protection but could be an alternative approach for prevention or treatment of UTI and recurrent UTI in the future. Our suggestion is to use the combination of the following methods to improve the efficacy of the DNA vaccine: cloning of cytokine genes as an adjuvant along with the immunogenic gene, developing the delivery methods, codon usage optimization, and using new vectors.
Figures and Tables
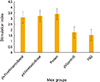 | Fig. 1Stimulation assay. There are significant response in DNA vaccine groups and protein group compared to control groups (pV and phosphate buffered saline [PBS]). |
 | Fig. 2Interferon-γ (IFN-γ), interleukin (IL)-12, IL-4, and IL-17 levels in spleen cell cultures of mice after 72 hours of stimulation with recombinant FimH antigen. The cytokine levels were determined using enzyme-linked immunosorbent assay. Data represent the means±standard deviations for triplicate culture of eight animals per group. IFN-γ (A) and IL-12 (B) showed significantly response in DNA vaccine group compare to control groups. IL-4 (C) and IL-17 levels (D) showed no significant response in DNA vaccine group compare to control. PBS, phosphate buffered saline. *p<0.05, **p<0.01, ***p<0.001. |
 | Fig. 3Challenge of mice with UPEC 35218. Two weeks after the last immunization, the bladders of mice (n=5) infected with 1×108 CFU of UPEC isolate. The levels of bladder colonization were determined 48 hours after challenge by plating tissue homogenates. Solid lines show median of colonization levels. Significant differences between groups were determined by Kruskal-Wallis analysis (Dunn's multiple comparison test) and are shown by brackets with asterisk. PBS, phosphate buffered saline. *p<0.05 was considered as significant. |
Notes
References
1. Klumpp DJ, Rycyk MT, Chen MC, Thumbikat P, Sengupta S, Schaeffer AJ. Uropathogenic Escherichia coli induces extrinsic and intrinsic cascades to initiate urothelial apoptosis. Infect Immun. 2006; 74:5106–5113.

2. Mysorekar IU, Mulvey MA, Hultgren SJ, Gordon JI. Molecular regulation of urothelial renewal and host defenses during infection with uropathogenic Escherichia coli. J Biol Chem. 2002; 277:7412–7419.

3. Blomgran R, Zheng L, Stendahl O. Uropathogenic Escherichia coli triggers oxygen-dependent apoptosis in human neutrophils through the cooperative effect of type 1 fimbriae and lipopolysaccharide. Infect Immun. 2004; 72:4570–4578.

4. Wright KJ, Seed PC, Hultgren SJ. Development of intracellular bacterial communities of uropathogenic Escherichia coli depends on type 1 pili. Cell Microbiol. 2007; 9:2230–2241.

5. Mulvey MA, Lopez-Boado YS, Wilson CL, et al. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science. 1998; 282:1494–1497.

6. Cirl C, Wieser A, Yadav M, et al. Subversion of Toll-like receptor signaling by a unique family of bacterial Toll/interleukin-1 receptor domain-containing proteins. Nat Med. 2008; 14:399–406.

7. Billips KA, Schaeffer J, Klumpp DJ. Molecular basis of uropathogenic Escherichia coli evasion of the innate immune response in the bladder. Infect Immun. 2008; 76:3891–3900.

8. Hunstad DA, Justice SS, Hung CS, Lauer SR, Hultgren SJ. Suppression of bladder epithelial cytokine responses by uropathogenic Escherichia coli. Infect Immun. 2005; 73:3999–4006.

9. Mulvey MA, Schilling JD, Martinez JJ, Hultgren SJ. Bad bugs and beleaguered bladders: interplay between uropathogenic Escherichia coli and innate host defenses. Proc Natl Acad Sci U S A. 2000; 97:8829–8835.

10. Sivick KE, Mobley HL. Waging war against uropathogenic Escherichia coli: winning back the urinary tract. Infect Immun. 2010; 78:568–585.

11. Ferraro B, Morrow MP, Hutnick NA, Shin TH, Lucke CE, Weiner DB. Clinical applications of DNA vaccines: current progress. Clin Infect Dis. 2011; 53:296–302.

12. Spearman P, Kalams S, Elizaga M, et al. Safety and immunogenicity of a CTL multiepitope peptide vaccine for HIV with or without GM-CSF in a phase I trial. Vaccine. 2009; 27:243–249.

13. Kutzler MA, Weiner DB. Developing DNA vaccines that call to dendritic cells. J Clin Invest. 2004; 114:1241–1244.

14. Bagherpour G, Fooladi AA, Mehrabadi JF, Nourani MR, Einollahi B. Evaluation of mammalian codon usage of fimH in DNA vaccine design. Acta Microbiol Immunol Hung. 2011; 58:259–271.

15. Johnson DE, Lockatell CV. Mouse model of ascending UTI involving short- and long-term indwelling catheters. In : Zak O, Sande MA, editors. Handbook of animal models of infection. Experimental models in antimicrobial chemotherapy. London: Academic Press;1999. p. 441–445.
16. Kim OY, Hong BS, Park KS, et al. Immunization with Escherichia coli outer membrane vesicles protects bacteria-induced lethality via Th1 and Th17 cell responses. J Immunol. 2013; 190:4092–4102.

17. Mulvey MA, Schilling JD, Hultgren SJ. Establishment of a persistent Escherichia coli reservoir during the acute phase of a bladder infection. Infect Immun. 2001; 69:4572–4579.

18. Schilling JD, Lorenz RG, Hultgren SJ. Effect of trimethoprim-sulfamethoxazole on recurrent bacteriuria and bacterial persistence in mice infected with uropathogenic Escherichia coli. Infect Immun. 2002; 70:7042–7049.

19. Goluszko P, Goluszko E, Nowicki B, Nowicki S, Popov V, Wang HQ. Vaccination with purified Dr Fimbriae reduces mortality associated with chronic urinary tract infection due to Escherichia coli bearing Dr adhesin. Infect Immun. 2005; 73:627–631.

20. O'Hanley P, Lark D, Falkow S, Schoolnik G. Molecular basis of Escherichia coli colonization of the upper urinary tract in BALB/c mice. Gal-Gal pili immunization prevents Escherichia coli pyelonephritis in the BALB/c mouse model of human pyelonephritis. J Clin Invest. 1985; 75:347–360.
21. Poggio TV, La Torre JL, Scodeller EA. Intranasal immunization with a recombinant truncated FimH adhesin adjuvanted with CpG oligodeoxynucleotides protects mice against uropathogenic Escherichia coli challenge. Can J Microbiol. 2006; 52:1093–1102.

22. Langermann S, Mollby R, Burlein JE, et al. Vaccination with FimH adhesin protects cynomolgus monkeys from colonization and infection by uropathogenic Escherichia coli. J Infect Dis. 2000; 181:774–778.

23. Supek F, Smuc T. On relevance of codon usage to expression of synthetic and natural genes in Escherichia coli. Genetics. 2010; 185:1129–1134.

24. Ko HJ, Ko SY, Kim YJ, Lee EG, Cho SN, Kang CY. Optimization of codon usage enhances the immunogenicity of a DNA vaccine encoding mycobacterial antigen Ag85B. Infect Immun. 2005; 73:5666–5674.

25. Tokuoka M, Tanaka M, Ono K, Takagi S, Shintani T, Gomi K. Codon optimization increases steady-state mRNA levels in Aspergillus oryzae heterologous gene expression. Appl Environ Microbiol. 2008; 74:6538–6546.

26. Uchijima M, Yoshida A, Nagata T, Koide Y. Optimization of codon usage of plasmid DNA vaccine is required for the effective MHC class I-restricted T cell responses against an intracellular bacterium. J Immunol. 1998; 161:5594–5599.
27. Burgess-Brown NA, Sharma S, Sobott F, Loenarz C, Oppermann U, Gileadi O. Codon optimization can improve expression of human genes in Escherichia coli: A multi-gene study. Protein Expr Purif. 2008; 59:94–102.

28. Wang Q, Mei C, Zhen H, Zhu J. Codon preference optimization increases prokaryotic cystatin C expression. J Biomed Biotechnol. 2012; 2012:732017.

29. Asadi Karam MR, Oloomi M, Mahdavi M, Habibi M, Bouzari S. Vaccination with recombinant FimH fused with flagellin enhances cellular and humoral immunity against urinary tract infection in mice. Vaccine. 2013; 31:1210–1216.

30. Titomirov AV, Sukharev S, Kistanova E. In vivo electroporation and stable transformation of skin cells of newborn mice by plasmid DNA. Biochim Biophys Acta. 1991; 1088:131–134.

31. Rosati M, Valentin A, Jalah R, et al. Increased immune responses in rhesus macaques by DNA vaccination combined with electroporation. Vaccine. 2008; 26:5223–5229.

32. Crottet P, Corthesy B. Secretory component delays the conversion of secretory IgA into antigen-binding competent F(ab')2: a possible implication for mucosal defense. J Immunol. 1998; 161:5445–5453.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download