Abstract
Although vaccination is regarded as one of the most effective public health measure to prevent and control infectious diseases, no vaccine is perfectly safe. Therefore, safety management is an essential component in running National Immunization Program. Here, we review the current issues and suggest future perspectives of Korean vaccine safety management system.
Immunization is among the most important public health measure to control and eliminate infectious diseases. However, safety of the vaccines and immunization is not perfect; therefore occurrence of adverse events following immunization (AEFI) is inevitable. Although most AEFIs are mild and does not cause serious health outcomes, some may cause substantial degree of disease and complication. Moreover, because immunization is given to the large population, the occurrence of serious AEFI, which is seen less in other medications which are used in treating disease, is expected.
Most vaccines that we use nowadays are not replaceable to another; therefore impetuous judgment on causality between certain vaccine and its AEFI may lead to negative impact on public health management on infectious diseases. Furthermore, false causality assessment of AEFI can cause misunderstanding of the public and lower their confidence on immunization safety. On another hand, disapproving such AEFI with ample amount of evidence is not acceptable in the practices of modern medicine. Therefore, scrupulous causality assessment between vaccine, immunization and AEFI should be conducted; and the investigation on the magnitude of safety of such vaccine; and considering its cost-benefit in the scope of public health should be in place in order to operate the National Immunization Program.
The AEFI can be classified as follows: by frequency (rare or common AEFI); by locality (local or systemic AEFI); by disease severity (mild or severe AEFI); and by pathophysiology. The stabilizers, immune potentiators, or antibiotics which are included in the vaccines may cause adverse reaction to susceptible vaccinees. Generally, these hyperreactive responses are postulated to occur because of egg-related antigen (measles, mumps, and rubella [MMR] and influenza vaccine), mercury (hepatitis B vaccine, diphtheria, tetanus, pertussis vaccine [DTaP], Japanese encephalitis vaccine, and influenza vaccine), vaccine stabilizer (MMR), or antibiotics included in the live-attenuated virus vaccine (MMR); however, these mechanisms are not clearly explained, often [1-4].
Most vaccines result in local reactions following vaccination such as pain, swelling and redness in some population; this frequency slightly increases following DTaP or tetanus-containing vaccines [5]. After 2-3 weeks of receiving Bacillus Calmette-Guérin (BCG) vaccine, localized inflammation and ulceration forms, then heal within few months [6]. Systemic reactions such as fever and malaise may occur in some population; the frequency is higher in DTaP, which may also result in irritability and decreased oral intake in infants [7]. Following MMR vaccination, systemic reactions such as measles-like illness due to vaccine-strain virus may occur on more than 5-10% of vaccinees [8]. This vaccine-related measles is generally mild in immune-competent persons; however, may result in severe disease in immune-compromised hosts, therefore MMR and other live-attenuated vaccines are contraindicated in such population. The attenuated mumps virus included in MMR may result arthralgia and lymphadenitis on less than 1% of those who are vaccinated [9].
The advances in vaccines and immunization practices have led to decline in the incidence of vaccine-preventable diseases worldwide. However, the increase in reported cases of AEFI has been noted recently, probably following increased utilization of vaccines; increased vaccinees who are susceptible to such complications; and increased awareness and notification. In the United States with long-standing history of AEFI surveillance system, the number of reported AEFI cases has exceeded the number of reported vaccine-preventable diseases; therefore the increase in public awareness on AEFI may occur, consequently [10].
In order to maintain the public confidence and to ensure political support on National Immunization Program, the public health sector must ensure the safety of vaccines and immunization practices. Before the introduction of AEFI surveillance system in the 1980s, neurologic adverse event such as seizure was reported following whole-cell pertussis vaccination in multiple countries. In response to the media coverage, immunization coverage decreased and then consequent resurgence of pertussis was observed in many countries such as Japan, Sweden, and UK [11,12]. In the US, lawsuits against vaccine manufacturers has resulted in renunciation of vaccine production, and therefore led to inadequate procurement of essential vaccines [13].
In order to minimize the unnecessary conflicts and arguments, and to achieve adequate level of immunization coverage in the population, the need for AEFI management system was raised [14]. The national AEFI management system was meant to distinguish between the true vaccine reactions and the coincidental events following immunization in timely manner, therefore to ensure safety of the vaccinees and to improve the confidence in National Immunization Program.
Most of other medications are intended to use for treatment purposes, however, vaccines are given to healthy people, especially children, to prevent the future or potential occurrence of infectious diseases. The unhealthy patients who are receiving the medications such as chemotherapeutic agents or antibiotics may tolerate the occurrence of adverse event. However, the vaccinees, who are mostly healthy without having any diseases, may not tolerate on reactions after they receive the vaccines. Furthermore, vaccines are forcibly given in some instances, therefore high level of safety should be guaranteed. In addition, most vaccines utilized by National Immunization Program are given to general populations in a large amount; therefore safety should be heavily reviewed.
In the Republic of Korea, AEFI management system began since 1994, after the two deaths following Japanese encephalitis vaccine immunization was reported [15]. In 2000, the system that included report of AEFI through Electronic Document Interchange was introduced, and all systems were computerized before 2001. In 2001, the Communicable Disease Control Act has mandated healthcare professionals to report all noticed AEFI to the public health centers, and in 2005, the system has introduced an Internet-based reporting system, which made the reporting more handy. Currently, vaccine safety management system in Korea is composed of four parts: rapid response system, AEFI surveillance system, AEFI investigation system, and vaccine injury compensation program (VICP) (Fig. 1). The Division of Vaccine Preventable Disease Control and National Immunization Program at Korea Centers for Disease Control and Prevention (KCDC) operates routine and ad-hoc surveillance measures on AEFI in Korea; performs epidemiological investigation on certain AEFIs such as serious adverse reactions or clustered AEFIs; and operates VICP.
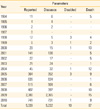
Since 1994, when 11 AEFIs were reported, the reported numbers for AEFI has increased to 741 in 2010 (Table 1). There were two surges in 2001 (n=141) and 2009 (n=2,380), when there were nationwide immunization campaign for measles and H1N1 influenza took place, respectively. Among 5,339 reported AEFIs between 1994 and 2010, 97 deaths and 10 disabled cases were notified.
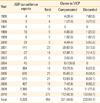
When classified according to vaccine types (Table 2), BCG was the vaccine with most AEFI cases reported in 2008 with 38.59 cases per 1,000,000 vaccinees. However, this rate decreased down to 10.38 in 2009 and 8.1 in 2010, when the government changed the vaccine-strain from Pasteur-strain to Danish-strain, because of the reactogenicity. In 2010, MMR and tetanus diphtheria vaccine (Td) were recognized as the second and third common vaccine to be reported as cause of AEFI.
When an AEFI has occurred, the vaccinees or their guardians may report the suspected AEFI through telephone to the local public health center; or by accessing website at http://nip.cdc.go.kr; and the healthcare professionals may report through telephone to the local public health center; or by accessing website at http://is.cdc.go.kr. When the local public health center receives the notification of suspected AEFI, then they report to the KCDC through website or by telephone. KCDC then compile the nationally-collected AEFI data, analyze the unexpected pattern of AEFI, and conducts overall management of vaccine safety in Korea.
Thorough investigation and clear description of serious AEFI quell unnecessary concerns regarding vaccine safety. However, the quality of data collected by the routine surveillance system is limited because data collected do not include detailed epidemiological or clinical information regarding AEFI.
In 2011, KCDC initiated to retrospectively investigate the vaccination-related serious AEFI of seasonal trivalent influenza in Korea from 2003 to 2010 [16]. During the period, 42 potentially serious AEFI were reported. Of those, nine Guillain-Barré syndrome (GBS), eighteen other neurologic events, eight local events, and seven miscellaneous events were included (Table 3). When classified according to causality assessment made by Korea Advisory Committee on Vaccine Injury Compensation for GBS, three were considered to be potentially associated to vaccination, whereas five were assessed as unlikely related. All of the three cellulitis cases had their injury sites that are not within 20 cm apart from the injection site, and two of the three cases had their symptoms onset after more than 2 weeks after vaccination. Approximately two third of localized events were assessed to have potential association with vaccination (62.5%, 5/8), whereas more than half of neurologic events (14/18) were considered to have no association with vaccination (unlikely).
In addition, ascertainment of each investigated cases is also important. In 2009-2010 season, mass vaccination for H1N1 pandemic influenza raised concern in a particular AEFI: the GBS. In 2011, KCDC investigated the data of reported cases of GBS following receipt of pandemic influenza vaccine in Korea [17]. All cases were reviewed under case definition developed by Brighton Collaboration Working Group, in order to ascertain a clear and precise case definition for AEFI data analysis. Of 29 reported cases during 2009-2010 season, 22 were confirmed to meet Brighton criteria level 1, 2, or 3 for GBS. Of those, 2 (9.1%) met level 1, 9 (40.9%) met level 2, and 11 (50.0%) met level 3. The Brighton case definition was used to improve the quality of AEFI data, and was applicable in retrospective review of medical records in cases with GBS after influenza A (H1N1) vaccination.
The compensation of vaccine injury was meant to decrease the negative public awareness on vaccine safety may decrease the population vaccination coverage, which then consequently decreases the population immunity. The Korea National Vaccine Injury Compensation Program (KVICP), which was established in 1994, compensates individuals who experience certain AEFI for vaccines that are recommended by the government. The program was first became a legal entity under the Prevention of Contagious Disease Act in 1994, and consists of Committee with a chairperson and specialists in pediatric, neurology, cardiology, epidemiology, forensic medicine, and pharmaceutical engineering. In order to be eligible for compensation, a claim must be filed within 5 years after occurrence of AEFI, and the patient must have spent more than KRW300,000 (approximately US$300) on health care expenses.
Table 4 depicts the annual number of potential adverse reactions that filed claim on VICP, and the number of cases compensated. Overall, nearly half of the cases were compen sated (48.6%). The proportion of compensation ranged from 25% (1996) to 100% (2004). Although the number of reported cases of AEFI has gradually increased during the last 10 years, the number of claims remained relatively constant during the same time-period (Table 4).
When a patient places a claim for compensation, the AEFI investigation team jointly operated by KCDC and local health authority starts an investigation. Determination of causal association between the injury and influenza vaccination is assessed by Korea Advisory Committee on Vaccine Injury Compensation, who use the simplified World Health Organization (WHO) categories of likelihood of causality: 1) definite, 2) probable, 3) possible, and 4) unlikely [18]. Compensations are made for cases that are classified as definite, probable, or possible.
The data collection and analysis of information on AEFI from the Korean AEFI surveillance system are continuously done. However, low reporting rate and limited data have prompted the introduction of supplementary surveillance measures to complete the data required to ensure vaccine safety in Korea. Further efforts will be needed to provide adequate data to ensure the safety of vaccines and immunizations currently practiced in the country. Many surveillance measures that are routinely performed or ad-hoc surveillance had showed the safety of vaccines currently utilized. Continual monitoring for AEFI is necessary to ensure the long-term safety of the vaccines, and to further contribute to vaccine policy for control of vaccine-preventable diseases and its complications in Korea.
Figures and Tables
Fig. 1
Structure of vaccine safety management in Korea. AEFI, adverse events following immunization.
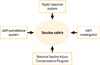
Table 2
Types of vaccines reported and reporting rate of adverse events following Immunization Surveillance System in Korea, 2008-2010

AEFI, adverse events following immunization; BCG, bacillus Calmette-Guerin; Hep-B, hepatitis B; DTaP-IPV, diphtheria, tetanus, pertussis vaccine and inactivated polio vaccine; MMR, measles, mumps and rubella vaccine; JEV, Japanese encephalitis vaccine; KHF, Korean hemorrhagic fever vaccine; Td, tetanus diphtheria vaccine.
References
1. Leventhal JS, Berger EM, Brauer JA, Cohen DE. Hypersensitivity reactions to vaccine constituents: a case series and review of the literature. Dermatitis. 2012. 23:102–109.
2. Forsdahl BA. Reactions of Norwegian children with severe egg allergy to an egg-containing influenza A (H1N1) vaccine: a retrospective audit. BMJ Open. 2012. 2:e000186.
3. Kelso JM, Jones RT, Yunginger JW. Anaphylaxis to measles, mumps, and rubella vaccine mediated by IgE to gelatin. J Allergy Clin Immunol. 1993. 91:867–872.

4. Clements CJ, McIntyre PB. When science is not enough: a risk/benefit profile of thiomersal-containing vaccines. Expert Opin Drug Saf. 2006. 5:17–29.

5. Shefer A, Dales L, Nelson M, Werner B, Baron R, Jackson R. Use and safety of acellular pertussis vaccine among adult hospital staff during an outbreak of pertussis. J Infect Dis. 1995. 171:1053–1056.

6. Verbov J. Local skin complications of BCG vaccination. Practitioner. 1984. 228:1069–1071.
7. Deloria MA, Blackwelder WC, Decker MD, et al. Association of reactions after consecutive acellular or whole-cell pertussis vaccine immunizations. Pediatrics. 1995. 96(3 Pt 2):592–594.

8. Jenkin GA, Chibo D, Kelly HA, Lynch PA, Catton MG. What is the cause of a rash after measles-mumps-rubella vaccination? Med J Aust. 1999. 171:194–195.

9. Virtanen M, Peltola H, Paunio M, Heinonen OP. Day-to-day reactogenicity and the healthy vaccinee effect of measles-mumps-rubella vaccination. Pediatrics. 2000. 106:E62.
10. Chen RT, Orenstein WA. Epidemiologic methods in immunization programs. Epidemiol Rev. 1996. 18:99–117.

11. Forsyth K, Nagai M, Lepetic A, Trindade E. Pertussis immunization in the global pertussis initiative international region: recommended strategies and implementation considerations. Pediatr Infect Dis J. 2005. 24:5 Suppl. S93–S97.
12. Galazka A. Control of pertussis in the world. World Health Stat Q. 1992. 45:238–247.
13. Cook KM, Evans G. The National Vaccine Injury Compensation Program. Pediatrics. 2011. 127:Suppl 1. S74–S77.

14. Looker C, Kelly H. No-fault compensation following adverse events attributed to vaccination: a review of international programmes. Bull World Health Organ. 2011. 89:371–378.

15. Kim BY, Kim DH, Lee HJ, et al. Investigation on the frequency and severity of common adverse reactions of Japanese encephalitis vaccines. Korean J Pediatr Infect Dis. 2009. 16:183–190.

16. Choe YJ, Cho H, Kim SN, Bae GR, Lee JK. Serious adverse events following receipt of trivalent inactivated influenza vaccine in Korea, 2003-2010. Vaccine. 2011. 29:7727–7732.

17. Choe YJ, Cho H, Bae GR, Lee JK. Guillain-Barre syndrome following receipt of influenza A (H1N1) 2009 monovalent vaccine in Korea with an emphasis on Brighton Collaboration case definition. Vaccine. 2011. 29:2066–2070.

18. Collet JP, MacDonald N, Cashman N, Pless R. Advisory Committee on Causality Assessment. Monitoring signals for vaccine safety: the assessment of individual adverse event reports by an expert advisory committee. Bull World Health Organ. 2000. 78:178–185.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download