Abstract
Purpose
There are different principles regarding varicella vaccination depending on social requirements. This study was performed to report a case of breakthrough chickenpox outbreak among a group of people in the healthcare center and also evaluate the seroprevalence of patients who have been managed with chronic neurological diseases in this center.
Materials and Methods
We included patients diagnosed with varicella in April 2009 as an index cases and investigated the past history for chickenpox and the varicella-specific IgG. Sixty-two patients (children) and 156 healthcare workers who may have had possible contact with the index cases were also investigated for.
Results
We investigated the seroprevalence of 62 patients not affected by the outbreak. The varicella vaccination rate in children was 90.3%. Sixty-one point three percent of all patients were seropositive and 63.6% of these patients were aged between 12 and 23 months and 87.5% were aged between 24 and 35 months. Seropositive rate was decreased for patients aged between 36 and 59 months while the seropositive prevalence has increased for patients over 5 years old. Over 90% of the adults investigated were seropositive. IgG seronegative despite vaccination was 32.1% (18 persons).
Conclusion
Breakthrough varicella outbreak can have huge impact in healthcare centers with affiliated group housing. We need to investigate vaccination status and immunogenicity according to ages and reflect appropriately on vaccination policy taking into consideration of social and medical requirements in the local area.
The varicella-zoster virus is transmitted by the airborne route. It is very contagious and found world-wide. It is a member of the family of alphaherpesvirus and typically causes a vesicular rash on the central areas such as the head, neck and trunk with fever [1]. Even with chickenpox, the mild form of primary infection to healthy children may cause complications like pneumonia, hepatitis, and even encephalitis. Complication risk is likely to be increased in older patients, immune-compromised hosts, and pregnant women. It is supposed that the varicella vaccines might protect 85% of chickenpox infection and 97% of severe secondary sequelae [2]. For these reasons, several countries adopted the varicella vaccine as a mandatory immunization program over time; 1986 in Japan, 1995 in the US, and 2005 in Korea [3,4]. In Korea, varicella vaccine is mandatory with the current protocol (established 2005) suggesting one dose between 12 and 15 months of age. If a child has no vaccination confirmed or no history of chickenpox before 13 years of age, one-dose of vaccine is recommended but if the child is over 13 years old without vaccine or chickenpox history, he or she is recommended for a two-dose schedule 4 to 8 weeks apart [5]. However, the unwanted phenomenon called "breakthrough varicella" or "mild varicella-like syndrome" emerged after the introduction of the varicella vaccine. It is defined as an infection after exposure to a wild-type virus strain in the previously vaccinated. Breakthrough varicella is known to have less than 3% incidence in vaccinated children after each year following vaccination. Even though most cases of breakthrough varicella are mild, it can pose a serious problem because of transmission risk [6-8]. In this article, we report a case of breakthrough outbreak of chickenpox among a group of people in a healthcare center and evaluate the seroprevalence of patients and health care workers who might have come in to contact with the index case.
We selected an initial patient with varicella diagnosed in April 2009 as an index case and investigated the past history for chickenpox and varicella vaccination. Serum samples were collected to look for varicella specific-IgM and IgG in 62 patients and 156 healthcare workers who were thought to have had a contact with the initialpatient. Enzyme immunoassay-for the qualitative detection and quantitative determination of specific-IgG antibodies to varicella-zoster virus was performed for each sample and we used Enzygnost anti-VZV IgM and IgG from Siemens (Marburg, Germany). The tests were based on VZV glycoproteins as antigens. In the result, greater than 1:40 was considered as positive for anti-IgM which implied acute infected or newly reactivated status and detectionover 50 IU/L was considered positive for anti-IgG which showed immunization had been successful.
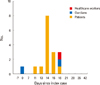
The Index case was a patient's mother. She complained of an itching rash on her son's second day in hospital. Her son was hospitalized for rehabilitation at the rehabilitation center, which was the communal center of concern. Right after nothing occurrence of chickenpox, they were quarantined with respiratory isolation. Since that, sixteen cases of outbreak were reported. Two adults including one healthcare worker and fourteen children were reported as having symptoms of varicella. All chickenpox cases had occurred within sixteen days after the index casewas recognized. Twelve out of fourteen children cases in children had received chickenpox vaccination or had a history of previous chickenpox exposure. Two children were confirmed as seropositive before they were diagnosed with chickenpox. Considering incubation period, the end of the outbreak was defined as when no more caseswere reported within three weeks from the last known case. The time-interval graph of cases after the index cases shown in Fig. 1.
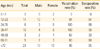
One hundred thirty young patients hospitalized at the same ward were identified and placed under cohort follow-up. Sixty two among them were analyzed for their seroprevalence. Among these 62 young patients, the total vaccination rate was 90.3%, IgG seropositivity was 61.3%, 63.6% of these patients were aged between 12 and 23 months and 18 persons were seronegative even though vaccination was performed (32.1%)(Table 1). In addition, we checked 156 health care workers at exposure risk. These patients self-reported a 70% immunization rate from vaccination or past infection and on investigation showed a positive seroprevalence rate of 96.2%. The seropositive rate was high until 12 months of age, then decreased by 5 years of age and again increased after that. The prevalence of seropositivity over five years old increased to over 90% in adults (Fig. 2).
Breakthrough varicella has become an emerging issue after the implementation of vaccine programsin modern times. Its symptoms include a maculopapular rash rather than the vesicular appearance which is found in typical varicella infection, fewer lesions (usually less than 50 lesions) compared to over 300 lesions in typical varicella and no fever [3,6,7]. Even though it manifests mild symptoms in healthy individuals, it may cause an outbreak in groups located in at-risk areas like health care centers or clustered facilities. Breakthrough varicella phenomenon can be explained through two ways; the first is the failure of the primary vaccine, which suggests the failure to produce a protective immune response to the vaccine. In a recent study for the etiology of breakthrough varicella, 113 children seroconverted out of 148 vaccines and 24% among them had no VZV antibodies detected after the one-shot vaccine [9]. Marin et al. [10] suggested that the lower seroconversion may have been caused by an absence of circulating wild type viruses which should have boosted host immunity in early life. In addition, there are other factors related to primary vaccine failure such as very young age on vaccination, the use of steroids, or immune-compromised states [11,12].
The second explanation of breakthrough varicella is the waning of immunity over time [13-15]. The level of protective efficacy in other studies has fallen from 97% to 84% at eight years after primary varicella immunization [16,17]. The Varicella Active Surveillance Project in California in 1995 to 2004 studied varicella surveillance cases concerning varicella vaccine status at least 42 days after immunization. One thousand eighty cases (9.5%) of breakthrough varicella were reported out of 11,356 varicella cases and among children ages between 8 and 12 years who had been vaccinated, children over 5 years after vaccination were more likely to have serious disease than children who were vaccinated less than 5 years previously. The annual rate of breakthrough varicella increased with the times after vaccination. Varicella infection had more cases in breakthrough infection than wild-type infection. However it did not mean there was increasing vaccine failure or breakthrough infection. Overall cases of varicella had decreased after vaccine program by about 85% in that period [18].
We reported that breakthrough varicella had occurred in most patients who had neurologic deficits and were receiving rehabilitative therapy. Depending on age, children had different vaccination histories but in most patients over one year of age, seroprevalence for varicella was highly controlled but decreasing antibody titers was seen. However, seroprevalence for varicella in adults has increased with ages. It is thought because they might have had asymptomatic contacts with wild-type varicella.
Even though breakthrough varicella has a milder manifestation of symptoms, it is still contagious and has a chance to create a more serious outbreak in high risk groups. Because of primary vaccine failure and waning of immunity, it needs to find another way to booster immunity against varicella. Additional dose of varicella vaccine can be one option. It is known to have an effect of stopping the spread and reducing the severity of symptoms [19] but as a national mandatory vaccination, the overall cost effectiveness should be considered. In our study, 15,000,000 KRW (about $130,000 US dollars) were used to perform serological studies and to control patients for management. To evaluate the efficacy of the vaccine, it needs to consider the management cost as well as the direct vaccine cost in situations of exposure. Even though varicella is known to show mild physical symptoms, it can still cause significant social and economic burdens.
The limitation in our study is following: The number of study was too small and study was performed in a specialized group so the result of the study could not be easily generalized to other groups or population. Also, from an immunological point of view, there were some cases who suffered from varicella infection despite having IgG against varicella. It might be explained that they had low titers of IgG against varicella or weaker protective effect on varicella infection even though they had IgG. Therefore, it needs to find a better way to represent for protective immunity against varicella. In addition, to increase the protective immunity, the second dose of varicella vaccine is adopted in several countries including the US but the effectiveness of the second dose of varicella vaccine is still short of evidence and needs to be studied more.
In conclusion, to achieve more effective control of varicella infection and to maintain protective immunity, further researches are required with standardized methods to evaluate infection control and more systemic data must be collected with multi-centered studies to bolster credibility. Also more studies about the effectiveness of an additional varicella vaccination are needed in the near future.
Figures and Tables
References
1. Gershon AA, Takahashi M, Seward JF. Varicella vaccine. In : Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. 6th ed. Philadelphia: Saunders Elsevier;2013. p. 837–869.
2. Vazquez M, LaRussa PS, Gershon AA, Steinberg SP, Freudigman K, Shapiro ED. The effectiveness of the varicella vaccine in clinical practice. N Engl J Med. 2001; 344:955–960.

3. Marin M, Guris D, Chaves SS, Schmid S, Seward JF. Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention (CDC). Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2007; 56:1–40.
4. Sadzot-Delvaux C, Rentier B, Wutzler P, et al. Varicella vaccination in Japan, South Korea, and Europe. J Infect Dis. 2008; 197:Suppl 2. S185–S190.

5. The Korean Pediatric Society. In : Lee HJ, editor. Varicella vaccine. Immunization guideline. 7th ed. Seoul: The Korean Pediatric Society;2012. p. 121–132.
6. White CJ, Kuter BJ, Ngai A, et al. Modified cases of chickenpox after varicella vaccination: correlation of protection with antibody response. Pediatr Infect Dis J. 1992; 11:19–23.
8. Seward JF, Zhang JX, Maupin TJ, Mascola L, Jumaan AO. Contagiousness of varicella in vaccinated cases: a household contact study. JAMA. 2004; 292:704–708.
9. Michalik DE, Steinberg SP, Larussa PS, et al. Primary vaccine failure after 1 dose of varicella vaccine in healthy children. J Infect Dis. 2008; 197:944–949.

10. Marin M, Watson TL, Chaves SS, et al. Varicella among adults: data from an active surveillance project, 1995-2005. J Infect Dis. 2008; 197:Suppl 2. S94–S100.

11. Salzman MB, Garcia C. Postexposure varicella vaccination in siblings of children with active varicella. Pediatr Infect Dis J. 1998; 17:256–257.

12. Black S, Ray P, Shinefield H, Saddier P, Nikas A. Lack of association between age at varicella vaccination and risk of breakthrough varicella, within the Northern California Kaiser Permanente Medical Care Program. J Infect Dis. 2008; 197:Suppl 2. S139–S142.

13. Seward JF, Zhang JX, Maupin TJ, Mascola L, Jumaan AO. Contagiousness of varicella in vaccinated cases: a household contact study. JAMA. 2004; 292:704–708.
14. Tugwell BD, Lee LE, Gillette H, Lorber EM, Hedberg K, Cieslak PR. Chickenpox outbreak in a highly vaccinated school population. Pediatrics. 2004; 113(3 Pt 1):455–459.

15. Lee BR, Feaver SL, Miller CA, Hedberg CW, Ehresmann KR. An elementary school outbreak of varicella attributed to vaccine failure: policy implications. J Infect Dis. 2004; 190:477–483.

16. Vazquez M, LaRussa PS, Gershon AA, et al. Effectiveness over time of varicella vaccine. JAMA. 2004; 291:851–855.

17. Vazquez M, Shapiro ED. Varicella vaccine and infection with varicella-zoster virus. N Engl J Med. 2005; 352:439–440.

18. Chaves SS, Gargiullo P, Zhang JX, et al. Loss of vaccine-induced immunity to varicella over time. N Engl J Med. 2007; 356:1121–1129.

19. American Academy of Pediatrics Committee on Infectious Diseases. Prevention of varicella: recommendations for use of varicella vaccines in children, including a recommendation for a routine two-dose varicella immunization schedule [Internet]. Elk Grove Village: American Academy of Pediatrics;2007. cited 2007 Apr 16. Available from: http://aapredbook.aappublications.org/site/news/Varicella-040907.pdf.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download