Abstract
Purpose
To prepare for vaccine shortages under an influenza pandemic, several antigen-sparing strategies have been investigated. This study was aimed to evaluate the immunogenicity of influenza vaccine at reduced intradermal and full intramuscular dose.
Materials and Methods
We compared the effect of one-fifth and one-half intradermal doses to the full intramuscular dose on immunogenicity in healthy young adults, using a commercial influenza vaccine. A hemagglutination inhibition assay was used to compare the immunogenicity of the vaccination methods.
Results
The one-fifth intradermal dose (3 µg hemagglutinin antigen, HA) was given to 30 participants, the one-half intradermal dose (7.5 µg HA) was given to 30, and the full intramuscular dose (15 µg HA) was given to 32. No significant differences among injection routes and dosages were seen for seroprotection rate, seroconversion rate, or geometric mean titer (GMT) fold-increase for A/H1N1, A/H3N2, and B at around 4 weeks from vaccination. Although GMT for influenza B was significantly lower at six months for the one-fifth intradermal vaccination compared to the full-dose intramuscular vaccination (32.8 vs. 63.2, p=0.048), all three groups met the Evaluation of Medicinal Products (EMA) immunogenicity criteria through 1 to 6 months.
Conclusion
Intradermal administration of a one-fifth dose of influenza vaccine elicited antibody responses comparable to the intradermal one-half dose and a conventional intramuscular vaccination at 1 month post-vaccination. The immunogenicity of the one-fifth intradermal dose was sufficient to meet the requirement for the EMA criteria at six months after influenza vaccination.
Influenza is one of the most common infectious diseases, with global epidemics occurring every 10-40 years. Although relatively benign, influenza can be associated with significant morbidity and mortality; influenza-related complications include pneumonia, encephalitis, myocarditis, and gradual deterioration of organ functions. Annual vaccination is the primary measure for preventing influenza-related morbidity and mortality, but the vaccine supply can be limited in an early pandemic situation. To prepare for potential vaccine shortages under an influenza pandemic, several antigen-sparing strategies have been investigated. These include adjuvanted vaccines and new methods of administering vaccines such as intradermal and subcutaneous injection.
Intradermal influenza vaccination requires less antigen to be as effective as regular intramuscular vaccination. The improved immune response is due to the higher level of dendritic cells in the dermis than in the muscle [1-3]. Furthermore, the dermis has a rich supply of blood and lymphatic vessels that aids circulation of immune cells [4,5].
In this study, we compared the long-term immunogenicity of intradermal vaccination at one-fifth and one-half doses to full-dose intramuscular vaccination of a commercial influenza vaccine in healthy young adults.
During the 2006-2007 influenza season, we compared the long-term immunogenicity in healthy young adults of an intradermal influenza vaccination at one-fifth and one-half the conventional antigenic contents with an intramuscular vaccination at the conventional dose (Fig. 1). We randomly assigned 96 participants in a 1:1:1 ratio to three vaccination groups: intradermal one-fifth dose (0.1 mL, 3 µg hemagglutinin antigen [HA]/strain), intradermal one-half (0.25 mL, 7.5 µg HA/strain), and intramuscular full-dose (0.5 mL, 15 µg HA/strain). Serum hemagglutinin inhibition (HI) antibody titers were determined for recommended influenza strains pre-vaccination and at one and six months after vaccination.
The trivalent inactivated split vaccine (Influenza HA vaccine, Green Cross, Yongin, Korea), containing 15 µg HA/strain per 0.5 mL dose, was used. The vaccine contained the following influenza strains: A/New Caledonia/20/99 (H1N1)-like virus, A/Wisconsin/67/2005 (H3N2)-like virus, and B/Malaysia/2506/2004-like virus.
Subjects enrolled in the study were healthy young adults aged 18-30 years. Exclusion criteria included immunosuppressant use, hypersensitivity to any vaccine component (including eggs), or a history of Guillain-Barre syndrome. Other exclusion criteria included thrombocytopenia, any coagulation disorder contraindicating intramuscular injection, or current febrile illness or another acute illness. Anyone who received gamma globulin during the previous three months or any other vaccination within the past 30 days was excluded.
The study was approved by the ethics committee of Korea University Guro Hospital (IRB No. KUGH0691) and was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice. All participants provided written, informed consent before enrollment. Venous blood samples of 10 mL were taken on day 0, post-vaccination day 30±7, and post-vaccination day 180±7.
Standard microtiter HI assays was performed as previously described [6]. A titer of ≥1:40 was considered a protective level [7,8]. Geometric mean titer (GMT) was determined pre-vaccination and at 1 month and 6 months post-vaccination. Serologic response, measured by HI antibody titer, was assessed using the criteria of the Evaluation of Medicinal Products (EMA) [9]. To confirm protective immunogenicity, at least one of the following three criteria were required for each influenza virus strain: 1) GMT-fold increase >2.5-fold, 2) Seroprotection rate >70%, and 3) Seroconversion rate >40%.
All statistical analysis was by SPSS version 15.0 (SPSS Inc., Chicago, IL, USA). Descriptive statistics are reported as number of participants and corresponding percentage. HI antibody titers are expressed as geometric mean with 95% confidence interval. Analysis of variance (ANOVA) was used to assess variation of GMTs among groups for each time point; multiple comparison tests were based on Turkey's method. Categorical variables were analyzed using chi-square test (Fisher's exact test for less than 30 samples). Statistical significance was signified by a p-value less than 0.05.
Among 96 enrolled participants, 4 were dropped from the study because of declining serum sampling. The experimental groups were comprised of 30, 30, and 32 participants in the intradermal one-fifth dose (0.1 mL, 3 µg HA), intradermal one-half dose (0.25 mL, 7.5 µg HA), and intramuscular full-dose (0.5 mL, 15 µg HA) groups, respectively. Baseline demographic characteristics of patients are in Table 1. No remarkable differences were observed between the groups.
All three groups met the EMA immunogenicity criteria in the 1-6 month study period (Table 2). For influenza A/H1N1 virus, the seroprotection (titer≥1:40) rates for the groups were 80.0% for the one-fifth dose, 90.0% for the one-half dose, and 87.5% for the full dose. For A/H3N2 virus, rates were 70.0% for the one-fifth dose, 90.0% for the one-half dose, and 84.4% for the full dose. For influenza B virus, rates were 60.0%, 60.0%, and 71.9%. No significant intergroup differences were observed at 1 month or 6 months post-vaccination (Table 2). GMTs increased by >2.5 in the 1-6 months for all 3 strains irrespective of injection route or dosage (Fig. 2). Seroconversion rates were above 40% for all three strains at 1 month post-vaccination, but the rate for influenza B declined to less than 40% at 6 months for the one-fifth dose vaccination (Table 2). GMT for influenza B was significantly lower at 6 months after the one-fifth intradermal dose compared to the full-dose intramuscular vaccination: 32.8 vs. 63.2 (p=0.048) (Table 2).
This study showed that the immunogenicity of one-fifth and one-half doses of intradermal seasonal influenza vaccine were largely comparable to full-dose intramuscular vaccination in healthy young adults. All three groups satisfied the EMA immunogenicity criteria for influenza vaccines, although the intradermal one-fifth dose vaccination elicited lower antibody responses than the intradermal one-half dose and the conventional dose of an intramuscular vaccination. Intramuscular vaccination bypasses the skin's immune system, while intradermal vaccination targets dense populations of dendritic cells in the skin. Skin is considered a desirable target for vaccination; about 25% of the skin is covered by dendritic cells, which are postulated to acquire antigens in peripheral tissue by priming naive T cells [1-3]. Moreover, antigen is suggested to become trapped in the cutaneous tissue for long periods after intradermal injection, compared to intramuscular injection [10].
Several trials of intradermal influenza vaccines have studied the characteristic features of the dermal immune system in adults of diverse ages [11,12]. A recent literature review [11] found 10 trials using reduced-dose intradermal influenza vaccines ranging from 3 to 9 µg HA per strain. Among these trials, five used a 3-µg dose [2,13-16]. Of the 10 trials, 8 found comparable responses between intradermal influenza vaccines at a reduced dose and a full-dose intramuscular vaccine [2,13,15-20]. One trial showed that a dose-sparing intradermal vaccine was superior to a conventional-dose intramuscular vaccine [21]. Thus, intradermal influenza vaccines at a reduced dose can be immunogenic in a way that is comparable to a full-dose intramuscular vaccine. However, data on antibody persistence has been insufficient. In this study, we showed that even a one-fifth dose of an intradermal vaccine induced antibody responses that persisted for six months.
This study has some limitations. First, we did not include elderly people, so long-term immunogenicity needs to be investigated among this population. Second, cross-reactive immunogenicity against drifted strains was not evaluated. Third, the history of influenza infections in the previous year was not considered.
Recently, the United States expanded the recommendation of influenza vaccination to children and adults without high-risk conditions [22]. In addition to these increased requirements, we experienced vaccine shortages during the 2009 influenza pandemic, and the United States suffered from vaccine shortages from the contamination of a vaccine-producing plant during the 2004-2005 season [23]. Thus, dose-sparing intradermal vaccines might be an effective strategy for preparation for an influenza pandemic, and could also be useful during interpandemic periods.
Figures and Tables
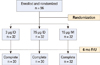 | Fig. 1A total of 96 healthy adults were enrolled in the study and assigned to one of three vaccination regimens, receiving intradermal (ID) one-fifth dose (0.1 mL, 3 ±g hemagglutinin antigen [HA]), ID one-half dose (0.25 mL, 7.5 ±g HA) or intramuscular (IM) full-dose (0.5 mL, 15 ±g HA) vaccination. Immunogenicity was assessed up to six months after vaccination. F/U, follow-up. |
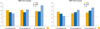 | Fig. 2Geometric mean titer (GMT) fold-increases were assessed at 1 month post-vaccination (A) and 6 months post-vaccination (B), by hemagglutination inhibition assay. |
References
1. Jakob T, Udey MC. Epidermal Langerhans cells: from neurons to nature's adjuvants. Adv Dermatol. 1999; 14:209–258.
2. Kenney RT, Frech SA, Muenz LR, Villar CP, Glenn GM. Dose sparing with intradermal injection of influenza vaccine. N Engl J Med. 2004; 351:2295–2301.

3. La Montagne JR, Fauci AS. Intradermal influenza vaccination: can less be more? N Engl J Med. 2004; 351:2330–2332.
4. Lambert PH, Laurent PE. Intradermal vaccine delivery: will new delivery systems transform vaccine administration? Vaccine. 2008; 26:3197–3208.

5. Nicolas JF, Guy B. Intradermal, epidermal and transcutaneous vaccination: from immunology to clinical practice. Expert Rev Vaccines. 2008; 7:1201–1214.

6. Cheong HJ, Song JY, Park JW, et al. Humoral and cellular immune responses to influenza vaccine in patients with advanced cirrhosis. Vaccine. 2006; 24:2417–2422.

7. Hobson D, Curry RL, Beare AS, Ward-Gardner A. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hyg (Lond). 1972; 70:767–777.

8. Davies JR, Grilli EA. Natural or vaccine-induced antibody as a predictor of immunity in the face of natural challenge with influenza viruses. Epidemiol Infect. 1989; 102:325–333.

9. European Committee for Proprietary Medicinal Products. Note for guidance on harmonisation of requirements for influenza vaccines. London: The Europen Agency for the Evaluation of Medicinal Products;1997.
10. Koff RS. Immunogenicity of hepatitis B vaccines: implications of immune memory. Vaccine. 2002; 20:3695–3701.

11. Young F, Marra F. A systematic review of intradermal influenza vaccines. Vaccine. 2011; 29:8788–8801.

12. Marra F, Young F, Richardson K, Marra CA. A meta-analysis of intradermal versus intramuscular influenza vaccines: immunogenicity and adverse events. Influenza Other Respi Viruses. 2013; 7:584–603.

13. Belshe RB, Newman FK, Cannon J, et al. Serum antibody responses after intradermal vaccination against influenza. N Engl J Med. 2004; 351:2286–2294.

14. Auewarakul P, Kositanont U, Sornsathapornkul P, Tothong P, Kanyok R, Thongcharoen P. Antibody responses after dose-sparing intradermal influenza vaccination. Vaccine. 2007; 25:659–663.

15. Van Damme P, Oosterhuis-Kafeja F, Van der Wielen M, Almagor Y, Sharon O, Levin Y. Safety and efficacy of a novel microneedle device for dose sparing intradermal influenza vaccination in healthy adults. Vaccine. 2009; 27:454–459.

16. Chuaychoo B, Wongsurakiat P, Nana A, Kositanont U, Maranetra KN. The immunogenicity of intradermal influenza vaccination in COPD patients. Vaccine. 2010; 28:4045–4051.

17. Belshe RB, Newman FK, Wilkins K, et al. Comparative immunogenicity of trivalent influenza vaccine administered by intradermal or intramuscular route in healthy adults. Vaccine. 2007; 25:6755–6763.

18. Beran J, Ambrozaitis A, Laiskonis A, et al. Intradermal influenza vaccination of healthy adults using a new microinjection system: a 3-year randomised controlled safety and immunogenicity trial. BMC Med. 2009; 7:13.

19. Arnou R, Eavis P, Pardo JR, Ambrozaitis A, Kazek MP, Weber F. Immunogenicity, large scale safety and lot consistency of an intradermal influenza vaccine in adults aged 18-60 years: randomized, controlled, phase III trial. Hum Vaccin. 2010; 6:346–354.

20. Chi RC, Rock MT, Neuzil KM. Immunogenicity and safety of intradermal influenza vaccination in healthy older adults. Clin Infect Dis. 2010; 50:1331–1338.

21. Leroux-Roels I, Vets E, Freese R, et al. Seasonal influenza vaccine delivered by intradermal microinjection: a randomised controlled safety and immunogenicity trial in adults. Vaccine. 2008; 26:6614–6619.

22. Campos-Outcalt D. CDC recommendations expand vaccine indications. J Fam Pract. 2009; 58:146–148.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download