Abstract
Purpose
Most of the atopic dermatitis (AD) patients and their parents refuse topical treatment because of concern about generalized side effect due to systemic absorption of topical corticosteroids. Therefore, a large number of studies reported difficulty in properly controlling in AD. However, investigations of the percutaneous absorption of topical corticosteroids are still insufficient.
Methods
One hundred nine patients who visited our atopy clinic and diagnosed as AD by a physician from January 2005 to January 2012 were enrolled. We examined serum corticosteroid (clobetasol propionate, hydrocortisone) level by liquid chromatography (LC) coupled with a tandem mass spectrometric (MS/MS) method.
Results
We developed the LC-MS/MS method to determine corticosteroids (clobetasol propionate, hydrocortisone) in sera of AD patients. Also, we confirmed precision, accuracy, limit of detection, limit of quantification, absolute recovery, and relative recovery of the experimental methods. We could not detect clobetasol propionate or hydrocortisone in sera of 109 AD patients using the newly developed LC-MS/MS method.
Conclusion
Regardless of age, the severity and illness duration of AD, clobetasol and hydrocortisone were not detected in sera. Although there are many other factors of determining systemic absorption of topical medications, our results showed that topical corticosteroids applied for several years in AD patients may be under the limit of detection in their sera by the LC-MS/MS method.
Go to : 
REFERENCES
1. Barbarot S, Bernier C, Deleuran M, De Raeve L, Eichenfield L, El Hachem M, et al. Therapeutic patient education in children with atopic dermatitis: position paper on objectives and recommendations. Pediatr Dermatol. 2013; 30:199–206.

2. Zuberbier T, Orlow SJ, Paller AS, Taïeb A, Allen R, Hernanz-Hermosa JM, et al. Patient perspectives on the management of atopic dermatitis. J Allergy Clin Immunol. 2006; 118:226–32.

3. Kojima R, Fujiwara T, Matsuda A, Narita M, Matsubara O, Nonoyama S, et al. Factors associated with steroid phobia in caregivers of children with atopic dermatitis. Pediatr Dermatol. 2013; 30:29–35.

4. Lee JY, Her Y, Kim CW, Kim SS. Topical corticosteroid phobia among parents of children with atopic eczema in Korea. Ann Dermatol. 2015; 27:499–506.

5. Hengge UR, Ruzicka T, Schwartz RA, Cork MJ. Adverse effects of topical glucocorticosteroids. J Am Acad Dermatol. 2006; 54:1–15.

6. Tempark T, Phatarakijnirund V, Chatproedprai S, Watcharasindhu S, Su-pornsilchai V, Wananukul S. Exogenous Cushing's syndrome due to topical corticosteroid application: case report and review literature. Endo-crine. 2010; 38:328–34.

7. Nieman LK. Consequences of systemic absorption of topical glucocorticoids. J Am Acad Dermatol. 2011; 65:250–2.

8. Park S, Cho GY, Lee SA, Kim HJ, Yum HY, Paeng KJ. Determination of corticosteroids in moisturizers by LC-MS/MS. Mass Spectom Lett. 2016; 7:26–9.

9. Rasmussen JE. Percutaneous absorption of topically applied triamcinolone in children. Arch Dermatol. 1978; 114:1165–7.

10. Turpeinen M, Salo OP, Leisti S. Effect of percutaneous absorption of hydrocortisone on adrenocortical responsiveness in infants with severe skin disease. Br J Dermatol. 1986; 115:475–84.

11. Turpeinen M, Mashkilleyson N, Björkstén F, Salo OP. Percutaneous absorption of hydrocortisone during exacerbation and remission of atopic dermatitis in adults. Acta Derm Venereol. 1988; 68:331–5.
12. Turpeinen M. Influence of age and severity of dermatitis on the percutaneous absorption of hydrocortisone in children. Br J Dermatol. 1988; 118:517–22.

13. Maibach HI, Wester RC. Issues in measuring percutaneous absorption of topical corticosteroids. Int J Dermatol. 1992; 31(Suppl 1):21–5.

Go to : 
Table 1.
Baseline characteristics of the study population (n=109)
| Characteristic | No. (%) |
|---|---|
| Age (yr) | |
| 0–1 | 12 (11.0) |
| 2–5 | 18 (16.5) |
| 6–10 | 19 (17.4) |
| 11–21 | 24 (22.0) |
| ≥22 | 36 (33.0) |
| SCORAD index | |
| Mild (≤15) | 15 (13.8) |
| Moderate (16–40) | 59 (54.1) |
| Severe (≥41) | 35 (32.1) |
Table 2.
Inter-, Intraday precision, accuracy of analytical method




 PDF
PDF ePub
ePub Citation
Citation Print
Print


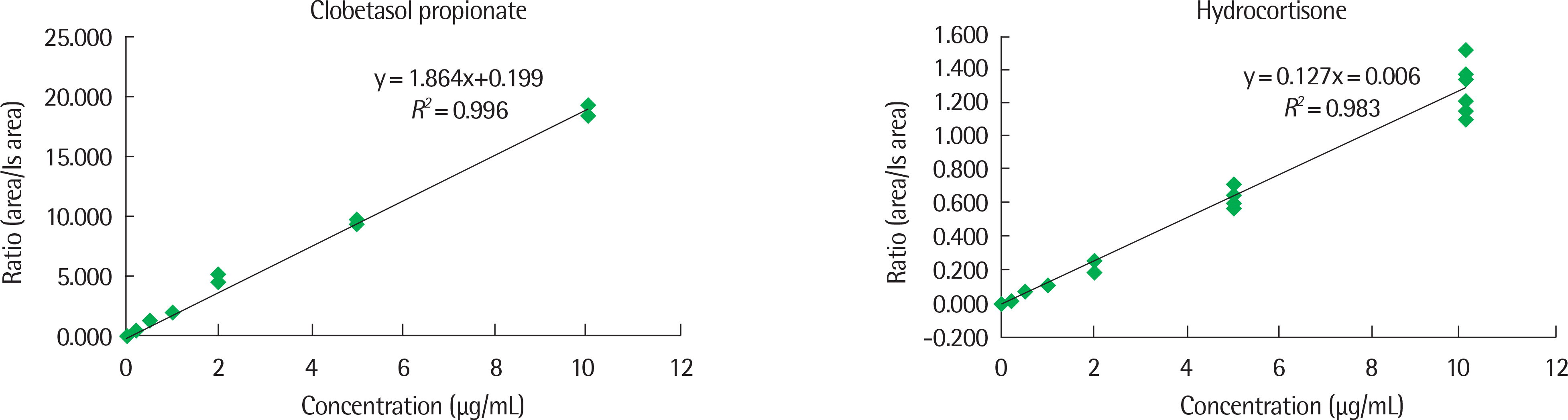
 XML Download
XML Download