Abstract
Purpose
Recent studies have shown that the cysteinyl leukotriene (cysLT) of exhaled breath condensate (EBC) could be predictive of inflammatory status and effectiveness of treatment in allergic disease. The aim of this study was to evaluate the inflammation and therapeutic effectiveness of cysLT in EBC in pediatric patients with allergic rhinitis (AR).
Methods
We enrolled 34 healthy children (median age, 4 years 10 months) and 67 AR children (median age, 5 years 1 month). All of the AR patients received intranasal steroid (fluticasone furoate) once daily for 2 weeks. After 2 week of fluticasone furoate treatment, they were classified into 2 groups: the fluticasone furoate (F) and montelukast (M) groups. We treated each group for another 8 weeks. To evaluate the therapeutic effectiveness, we used symptom score (SS) and EBC leukotriene E4 (LTE4). EBC samples were collected with RTube. Each parameter was checked at 0, 2, and 10 weeks of therapy.
Results
Most of the AR patients showed clinical improvement with 2- and 10-week fluticasone therapy (F group: 0-week SS, 5.6; 2-week SS, 3.6; 10-week SS, 2.1; P<0.01; M group: 0-week SS, 4.8; 2-week SS, 3.2; 10-week SS, 1.9: P<0.01). LTE4 levels were higher in AR patients than in control subjects (0 week: 87 pg/mL vs. 18 pg/mL) and were reduced after 2 weeks of fluticasone treatment (F group: 90→51.6 pg/mL, P<0.01; M group: 84→46.1 pg/mL, P<0.01). After 10 weeks of treatment, there was no significant difference in the LTE4 level between the F and M groups.
Figures and Tables
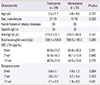
Table 1
Characteristics of the allergic rhinitis and control group

Table 2
Characteristics of the 2 treatment groups
References
1. Lee SI, Shin MH, Lee HB, Lee JS, Son BK, Koh YY, et al. Prevalences of symptoms of asthma and other allergic diseases in korean children: a nationwide questionnaire survey. J Korean Med Sci. 2001; 16:155–164.

2. Hong SJ, Lee MS, Sohn MH, Shim JY, Han YS, Park KS, et al. Self-reported prevalence and risk factors of asthma among Korean adolescents: 5-year follow-up study, 1995-2000. Clin Exp Allergy. 2004; 34:1556–1562.

3. Ahn K, Kim J, Kwon HJ, Chae Y, Hahm MI, Lee KJ, et al. The prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in Korean children: nationawide cross-sectional survey using complex sampling design. J Korean Med Assoc. 2011; 54:769–778.

5. Meltzer EO. Role for cysteinyl leukotriene receptor antagonist therapy in asthma and their potential role in allergic rhinitis based on the concept of “one linked airway disease”. Ann Allergy Asthma Immunol. 2000; 84:176–185.

6. Bachert C, Vignola AM, Gevaert P, Leynaert B, Van Cauwenberge P, Bousquet J. Allergic rhinitis, rhinosinusitis, and asthma: one airway disease. Immunol Allergy Clin North Am. 2004; 24:19–43.

7. Gibson PG, Henry RL, Thomas P. Noninvasive assessment of airway inflammation in children: induced sputum, exhaled nitric oxide, and breath condensate. Eur Respir J. 2000; 16:1008–1015.
8. Profita M, La Grutta S, Carpagnano E, Riccobono L, Di Giorgi R, Bonanno A, et al. Noninvasive methods for the detection of upper and lower airway inflammation in atopic children. J Allergy Clin Immunol. 2006; 118:1068–1074.

9. Hunt J. Exhaled breath condensate: an evolving tool for noninvasive evaluation of lung disease. J Allergy Clin Immunol. 2002; 110:28–34.

10. Cáp P, Pehal F, Chládek J, Malý M. Analysis of exhaled leukotrienes in nonasthmatic adult patients with seasonal allergic rhinitis. Allergy. 2005; 60:171–176.

11. Serrano CD, Valero A, Bartra J, Roca-Ferrer J, Muñoz-Cano R, Sánchez-López J, et al. Nasal and bronchial inflammation after nasal allergen challenge: assessment using noninvasive methods. J Investig Allergol Clin Immunol. 2012; 22:351–356.
12. Rosias PP, Dompeling E, Hendriks HJ, Heijnens JW, Donckerwolcke RA, Jöbsis Q. Exhaled breath condensate in children: pearls and pitfalls. Pediatr Allergy Immunol. 2004; 15:4–19.

13. Sandrini A, Ferreira IM, Jardim JR, Zamel N, Chapman KR. Effect of nasal triamcinolone acetonide on lower airway inflammatory markers in patients with allergic rhinitis. J Allergy Clin Immunol. 2003; 111:313–320.

14. Vass G, Huszár E, Augusztinovicz M, Baktai G, Barát E, Herjavecz I, et al. The effect of allergic rhinitis on adenosine concentration in exhaled breath condensate. Clin Exp Allergy. 2006; 36:742–747.

15. Davidsson A, Soderstrom M, Sjosward KN, Schmekel B. Chlorine in breath condensate: a measure of airway affection in pollinosis? Respiration. 2007; 74:184–191.

16. Failla M, Biondi G, Provvidenza Pistorio M, Gili E, Mastruzzo C, Vancheri C, et al. Intranasal steroid reduces exhaled bronchial cysteinyl leukotrienes in allergic patients. Clin Exp Allergy. 2006; 36:325–330.

17. Scichilone N, Arrigo R, Paternò A, Santagata R, Impellitteri S, Braido F, et al. The effect of intranasal corticosteroids on asthma control and quality of life in allergic rhinitis with mild asthma. J Asthma. 2011; 48:41–47.

18. Vass G, Huszár E, Barát E, Valyon M, Kiss D, Pénzes I, et al. Comparison of nasal and oral inhalation during exhaled breath condensate collection. Am J Respir Crit Care Med. 2003; 167:850–855.

19. Zagórska W, Grzela K, Kulus M, SobczyXMLLink_XYZski M, Grzela T. Increased cys-leukotrienes in exhaled breath condensate and decrease of PNIF after intranasal allergen challenge support the recognition of allergic rhinitis in children. Arch Immunol Ther Exp (Warsz). 2013; 61:327–332.

20. Tanou K, Koutsokera A, Kiropoulos TS, Maniati M, Papaioannou AI, Georga K, et al. Inflammatory and oxidative stress biomarkers in allergic rhinitis: the effect of smoking. Clin Exp Allergy. 2009; 39:345–353.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download