Abstract
Purpose
Previous studies have reported that clinical efficacy of steroid therapy for acute bronchiolitis is controversial. However, since it is still frequently used in clinical practice, we sought to re-evaluate its effectiveness.
Methods
This study included 277 children with acute bronchiolitis who were admitted to Kyungpook National University Children's Hospital from March 2013 to July 2016. Erythrocyte sedimentation rates, C-reactive protein (CRP) levels, and viral polymerase chain reaction testing results were obtained, and respiratory rate (RR) was measured periodically. Forty-eight patients were treated with an intravenous (IV) steroid (17.3%, IV group) and 19 patients were treated with a per oral (PO) steroid medication (6.9%, PO group). The remaining 210 patients were steroid-free patients (74.2%, nonsteroid group).
Results
RR and CRP levels were higher in the IV group, along with a longer hospitalization period and duration of wheezing. The rate of change from the fastest initial RR to the mean RR on the first treatment day was greatest in the IV group; this finding was statistically significant after controlling for initial RR (16.06% in the IV group, 3.94% in the PO group, 4.90% in the nonsteroid group; P<0.01).
Figures and Tables
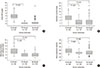 | Fig. 1(A) The mean value of initial CRP in the IV steroid group was higher than PO steroid group. (B) The mean duration of admission in the group of IV steroid was longer than other 2 groups. (C) The mean time of wheezing resolution after admission in IV steroid group was longer than other 2 groups. (D) The mean of initial respiratory rate in the first day of therapy in IV steroid group was faster than other 2 groups. CRP, C-reactive protein; IV, intravenous; PO, per oral. |
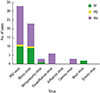 | Fig. 2The result of multiplex real-time polymerase chain reaction (RT-PCR) detection test (n=103) shows respiratory syncytial virus (RSV) is the most common in any group. IV, intravenous; PO, per oral. |
 | Fig. 3The rate of change of respiratory rate (RR) between the fastest RR before treatment and the mean RR of first day of treatment was higher in intravenous (IV) steroid group than other 2 groups. PO, per oral. |
Table 1
Characteristics of the subjects

References
1. Wright A. The Tucson children's respiratory study. II: lower respiratory tract illness in the first year of life. Pediatr Infect Dis J. 1990; 9:686.
2. Hall CB, Weinberg GA, Iwane MK, Blumkin AK, Edwards KM, Staat MA, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009; 360:588–598.

3. Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010; 375:1545–1555.

4. Kim HJ, Kim JH, Kang IJ. Association of respiratory viral infection and atopy with severity of acute bronchiolitis in infants. Pediatr Allergy Respir Dis. 2011; 21:302–312.

5. Shay DK, Holman RC, Newman RD, Liu LL, Stout JW, Anderson LJ. Bronchiolitis-associated hospitalizations among US children, 1980-1996. JAMA. 1999; 282:1440–1446.

6. Ralston SL, Lieberthal AS, Meissner HC, Alverson BK, Baley JE, Gadomski AM, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014; 134:e1474–e1502.

7. Castro-Rodriguez JA, Rodriguez-Martinez CE, Sossa-Briceño MP. Principal findings of systematic reviews for the management of acute bronchiolitis in children. Paediatr Respir Rev. 2015; 16:267–275.

8. Baumer JH. SIGN guideline on bronchiolitis in infants. Arch Dis Child Educ Pract Ed. 2007; 92:ep149–ep151.

9. Turner T, Wilkinson F, Harris C, Mazza D. Health for Kids Guideline Development Group. Evidence based guideline for the management of bronchiolitis. Aust Fam Physician. 2008; 37(6 Spec No):6–13.
10. Kim JS, In DK, Sun YH, Hong HJ, Cho KH, Son DW, et al. Current management of acute bronchiolitis in Incheon. Pediatr Allergy Respir Dis. 2006; 16:150–161.
11. Patel H, Platt R, Lozano JM, Wang EE. Glucocorticoids for acute viral bronchiolitis in infants and young children. Cochrane Database Syst Rev. 2004; (3):CD004878.

12. Garrison MM, Christakis DA, Harvey E, Cummings P, Davis RL. Systemic corticosteroids in infant bronchiolitis: a meta-analysis. Pediatrics. 2000; 105:E44.

13. Blom DJ, Ermers M, Bont L, van Woensel JB, Van Aalderen WM. WITHDRAWN: inhaled corticosteroids during acute bronchiolitis in the prevention of post-bronchiolitic wheezing. Cochrane Database Syst Rev. 2011; (1):CD004881.
14. Hartling L, Fernandes RM, Bialy L, Milne A, Johnson D, Plint A, et al. Steroids and bronchodilators for acute bronchiolitis in the first two years of life: systematic review and meta-analysis. BMJ. 2011; 342:d1714.

15. Bunch C, Hoffman R. Prednisone drugdex drug evaluation. Micromedex Healthcare Ser. 1998; 100.
16. Katzung BG. Basic and clinical pharmacology. Stamford (CT): Appleton & Lange;1998.
17. Thomas LH, Stott EJ, Collins AP, Crouch S, Jebbett J. Infection of gnotobiotic calves with a bovine and human isolate of respiratory syncytial virus. Modification of the response by dexamethasone. Arch Virol. 1984; 79:67–77.

18. Guilbert TW, Morgan WJ, Zeiger RS, Bacharier LB, Boehmer SJ, Krawiec M, et al. Atopic characteristics of children with recurrent wheezing at high risk for the development of childhood asthma. J Allergy Clin Immunol. 2004; 114:1282–1287.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download