Abstract
Purpose
Croup, a common childhood respiratory illness with various severities, has many unanswered questions. Laryngotracheobronchopneumonitis (LTBP) is a disease entity considered to be an extension of croup to the lower respiratory tract. The object of this study was to compare epidemiology, clinical characteristics, and viral etiologic spectrum between croup and LTBP.
Methods
Patients hospitalized with croup at Gachon University Gil Hospital from January 2010 to April 2016 were recruited. LTBP was defined as pneumonia confirmed on radiographs of patients with croup. Clinical findings and demographic data were reviewed of patients whose nasopharyngeal swabs were done for viral analysis.
Results
A total of 371 patients with only croup and 63 patients with LTBP were included. Croup was found to be significantly associated with parainfluenza virus type 1 (P=0.006). LTBP was related to parainfluenza virus type 3, respiratory syncytial virus, and human bocavirus (P=0.001, P=0.030, and P=0.019, respectively). The duration of fever was longer in patients with LTBP than in those with croup (3.87±1.85 days vs. 2.86±1.80 days, P<0.001).
Go to : 
REFERENCES
1. Cherry JD. Croup (laryngitis, laryngotracheitis, spasmodic croup, laryn-gotracheobronchitis, bacterial tracheitis, and laryngotracheobronchopneumonitis). Feigin RD, Cherry J, Demmler-Harrison GJ, Kaplan SL, editors. Textbook of pediatric infectious diseases. 5th ed.Philadelphia (PA): Elsevier;2004. p. 252–65.

3. Lee DR, Lee CH, Won YK, Suh DI, Roh EJ, Lee MH, et al. Clinical characteristics of children and adolescents with croup and epiglottitis who visited 146 Emergency Departments in Korea. Korean J Pediatr. 2015; 58:380–5.

4. Miranda AD, Valdez TA, Pereira KD. Bacterial tracheitis: a varied entity. Pediatr Emerg Care. 2011; 27:950–3.
5. Durward AD, Nicoll SJ, Oliver J, Tibby SM, Murdoch IA. The outcome of patients with upper airway obstruction transported to a regional paediatric intensive care unit. Eur J Pediatr. 1998; 157:907–11.

6. Roosevelt G. Acute inflammatory upper airway obstruction. Kliegman RM, Stanton BF, St. Geme JW III, Schor NF, Behrman RE, editors. Nelson textbook of pediatrics. 20th ed.Philadelphia: Elsevier;2016. p. 2031–6.
7. Cherry JD. Clinical practice. Croup. N Engl J Med. 2008; 358:384–91.
8. Bernstein T, Brilli R, Jacobs B. Is bacterial tracheitis changing? A 14-month experience in a pediatric intensive care unit. Clin Infect Dis. 1998; 27:458–62.

9. Leung AK, Kellner JD, Johnson DW. Viral croup: a current perspective. J Pediatr Health Care. 2004; 18:297–301.

10. Denny FW, Murphy TF, Clyde WA Jr, Collier AM, Henderson FW. Croup: an 11-year study in a pediatric practice. Pediatrics. 1983; 71:871–6.

11. Sung JY, Lee HJ, Eun BW, Kim SH, Lee SY, Lee JY, et al. Role of human coronavirus NL63 in hospitalized children with croup. Pediatr Infect Dis J. 2010; 29:822–6.

12. Castro-Rodriguez JA, Holberg CJ, Morgan WJ, Wright AL, Halonen M, Taussig LM, et al. Relation of two different subtypes of croup before age three to wheezing, atopy, and pulmonary function during childhood: a prospective study. Pediatrics. 2001; 107:512–8.
13. Downham MA, McQuillin J, Gardner PS. Diagnosis and clinical significance of parainfluenza virus infections in children. Arch Dis Child. 1974; 49:8–15.

14. Peltola V, Heikkinen T, Ruuskanen O. Clinical courses of croup caused by influenza and parainfluenza viruses. Pediatr Infect Dis J. 2002; 21:76–8.

15. Yang SI, Rho JH, Sun YH, Cho KH, Shim SY, Eun BW, et al. The comparison of clinical characteristics and courses of pediatric patients hospitalized with pandemic influenza A (H1N1) and seasonal influenza from 2009 to 2011. Pediatr Allergy Respir Dis. 2012; 22:292–301.

16. van der Hoek L, Sure K, Ihorst G, Stang A, Pyrc K, Jebbink MF, et al. Croup is associated with the novel coronavirus NL63. PLoS Med. 2005; 2:e240.

17. Wu PS, Chang LY, Berkhout B, van der Hoek L, Lu CY, Kao CL, et al. Clinical manifestations of human coronavirus NL63 infection in children in Taiwan. Eur J Pediatr. 2008; 167:75–80.

Go to : 
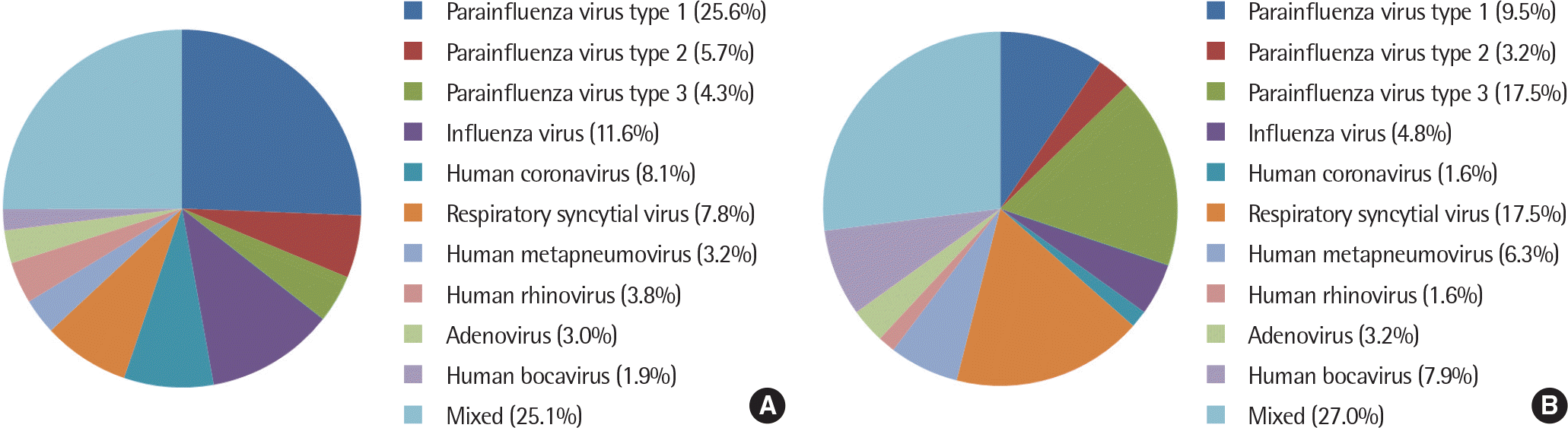 | Fig. 1.Distribution of etiologic agents of hospitalized pediatric patients with croup (A) and laryngotracheobronchopneumonitis (B). |
Table 1.
Patient demographics
Table 2.
Clinical characteristics of the study subjects
| Variable | Croup (n=371) | LTBP (n=63) | P-value |
|---|---|---|---|
| Fever | 330 (88.9) | 60 (95.2) | 0.174 |
| Barking cough | 298 (80.3) | 49 (77.8) | 0.613 |
| Respiratory difficulty | 231 (62.4) | 30 (47.6) | 0.036∗ |
| Voice change | 259 (69.8) | 42 (66.7) | 0.657 |
| Sputum | 246 (66.3) | 41 (65.1) | 0.886 |
| Rhinorrhea | 241 (65.0) | 44 (69.8) | 0.477 |
| Pharyngeal injection | 235 (63.3) | 34 (54.0) | 0.163 |
| Chest retraction | 83 (22.4) | 13 (20.6) | 0.870 |
| Wheezing | 41 (11.1) | 8 (12.7) | 0.669 |
| Crackle | 35 (9.4) | 12 (19.0) | 0.029∗ |
| Stridor | 223 (60.1) | 36 (57.1) | 0.678 |
Table 3.
Laboratory findings of hospitalized pediatric patients with croup and LTBP
| Variable | Croup (n=371) | LTBP (n=63) | P-value |
|---|---|---|---|
| WBC (×10³ cell/μL) | 12.07±5.21 | 11.63±4.62 | 0.529 |
| Neutrophil (%) | 46.39±17.33 | 51.69±16.45 | 0.024∗ |
| Lymphocyte (%) | 41.32±16.08 | 36.79±14.72 | 0.037∗ |
| Platelet (×10³ cells/μL) | 280.60±92.21 | 292.71±93.01 | 0.336 |
| CRP (mg/dL) | 1.30±1.59 | 1.74±1.81 | 0.045∗ |
| ESR (mm/hr) | 14.37±11.30 | 18.42±15.14 | 0.146 |
Table 4.
Clinical courses of hospitalized pediatric patients with croup and LTBP
| Variable | Croup (n=371) | LTBP (n=63) | P-value |
|---|---|---|---|
| Duration of hospitalization (day) | 4.36±1.42 | 5.29±1.90 | <0.001∗ |
| Total fever duration (day) | 2.86±1.80 | 3.87±1.85 | <0.001∗ |
| High fever (≥39˚C) | 204 (55.0) | 40 (63.5) | 0.220 |
| O2 supplementation | 83 (22.4) | 11 (17.5) | 0.508 |
| ICU admission (intubated) | 1 (0.3) | 1 (1.6) | 0.268 |
| Inhaled epinephrine | 305 (82.2) | 51 (81.0) | 1.000 |
| Antibiotics | 220 (59.3) | 40 (63.5) | 0.580 |
| Systemic steroid treatment | 238 (64.2) | 37 (58.7) | 0.480 |
Table 5.
Etiologic agents in hospitalized pediatric patients with croup and LTBP
| Virus | Odds ratio | 95% CI | P-value |
|---|---|---|---|
| Parainfluenza virus type 1 | 0.31 | 0.13–0.73 | 0.006∗ |
| Parainfluenza virus type 2 | 0.55 | 0.13–2.39 | 0.554 |
| Parainfluenza virus type 3 | 4.69 | 2.07–10.67 | 0.001∗ |
| Influenza virus | 0.38 | 0.12–1.27 | 0.123 |
| Human coronavirus | 0.18 | 0.03–1.37 | 0.066 |
| Respiratory syncytial virus | 2.49 | 1.18–5.29 | 0.030∗ |
| Human metapneumovirus | 2.03 | 0.63–6.50 | 0.268 |
| Human rhinovirus | 0.41 | 0.05–3.18 | 0.707 |
| Adenovirus | 1.07 | 0.23–4.96 | 0.586 |
| Human bocavirus | 4.48 | 1.38–14.59 | 0.019∗ |
| Mixed | 1.11 | 0.60–2.02 | 0.755 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download