Abstract
Diagnostic methods for drug allergy include the patient's history, in vivo skin test, in vitro laboratory test, and provocation test. How-ever, the history is often not reliable, procedures for in vivo and in vitro tests are not standardized, and provocation tests are some-times harmful to patients. Generally, skin prick and intradermal tests are useful for immediate reactions; in contrast, patch test and delayed reading of both skin prick and intradermal tests are helpful for delayed reactions. A drug provocation test is the gold standard for both responses, and it is necessary to be aware of exact indications and contraindications with appropriate drugs, doses, and intervals. To date, several methods have been developed to detect culprit agents for drug hypersensitivity reactions, but they are neither completely well validated nor standardized. Based on this awareness and necessity, the Korean Academy of Asthma, Allergy and Clinical Immunology launched the Standardization Committee to review the international guidelines and the literature, and then developed the consensus report on the procedures and applications of diagnostic tests for drug allergy.
REFERENCES
1. International drug monitoring: the role of national centres. Report of a WHO meeting. World Health Organ Tech Rep Ser. 1972; 498:1–25.
2. Joint Task Force on Practice Parameters; American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology; Joint Council of Allergy, Asthma and Immunology. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010; 105:259–73.
3. Barbaud A, Gonçalo M, Bruynzeel D, Bircher A. European Society of Contact Dermatitis. Guidelines for performing skin tests with drugs in the investigation of cutaneous adverse drug reactions. Contact Dermatitis. 2001; 45:321–8.
4. Brockow K, Romano A, Blanca M, Ring J, Pichler W, Demoly P. General considerations for skin test procedures in the diagnosis of drug hyper-sensitivity. Allergy. 2002; 57:45–51.

5. Aberer W, Bircher A, Romano A, Blanca M, Campi P, Fernandez J, et al. Drug provocation testing in the diagnosis of drug hypersensitivity reactions: general considerations. Allergy. 2003; 58:854–63.

6. Johansen JD, Aalto-Korte K, Agner T, Andersen KE, Bircher A, Bruze M, et al. European Society of Contact Dermatitis guideline for diagnostic patch testing - recommendations on best practice. Contact Dermatitis. 2015; 73:195–221.

7. Brockow K, Garvey LH, Aberer W, Atanaskovic-Markovic M, Barbaud A, Bilo MB, et al. Skin test concentrations for systemically administered drugs – an ENDA/EAACI Drug Allergy Interest Group position paper. Allergy. 2013; 68:702–12.

8. Cho YJ, Sohn SW, Kang HR, Kim SH, Kim JH, Kim TB, et al. Guideline for antibiotic skin test - for immediate hypersensitivity reaction. Seoul: Medrang;2012.
9. Torres MJ, Blanca M, Fernandez J, Romano A, Weck A, Aberer W, et al. Diagnosis of immediate allergic reactions to beta-lactam antibiotics. Allergy. 2003; 58:961–72.

10. Mangodt EA, Van Gasse AL, Decuyper I, Uyttebroek A, Faber MA, Saba-to V, et al. In vitro diagnosis of immediate drug hypersensitivity: should we go with the flow. Int Arch Allergy Immunol. 2015; 168:3–12.

11. Kränke B, Aberer W. Skin testing for IgE-mediated drug allergy. Immunol Allergy Clin North Am. 2009; 29:503–16.

12. Won HK, Yang MS, Song WJ, Kim SH, Park HW, Chang YS, et al. Determination of nonirritating concentrations of antibiotics for intradermal skin tests in Korean adults. J Allergy Clin Immunol Pract. 2017; 5:192–4. e2.

13. Alanko K. Topical provocation of fixed drug eruption. A study of 30 patients. Contact Dermatitis. 1994; 31:25–7.

14. Ozkaya-Bayazit E, Bayazit H, Ozarmagan G. Topical provocation in 27 cases of cotrimoxazole-induced fixed drug eruption. Contact Dermatitis. 1999; 41:185–9.
15. Brajon D, Menetre S, Waton J, Poreaux C, Barbaud A. Non-irritant concentrations and amounts of active ingredient in drug patch tests. Contact Dermatitis. 2014; 71:170–5.

16. Brockow K, Romano A. Skin tests in the diagnosis of drug hypersensitivity reactions. Curr Pharm Des. 2008; 14:2778–91.

17. Nugent JS, Quinn JM, McGrath CM, Hrncir DE, Boleman WT, Freeman TM. Determination of the incidence of sensitization after penicillin skin testing. Ann Allergy Asthma Immunol. 2003; 90:398–403.

18. Aberer W, Kränke B. Provocation tests in drug hypersensitivity. Immunol Allergy Clin North Am. 2009; 29:567–84.

19. Executive summary of disease management of drug hypersensitivity: a practice parameter. Joint Task Force on Practice Parameters, the American Academy of Allergy, Asthma and Immunology, the American Academy of Allergy, Asthma and Immunology, and the Joint Council of Allergy, Asthma and Immunology. Ann Allergy Asthma Immunol. 1999; 83(6 Pt 3):665–700.
20. Kim YJ, Lim KH, Kim MY, Jo EJ, Lee SY, Lee SE, et al. Cross-reactivity to acetaminophen and celecoxib according to the type of nonsteroidal anti-inflammatory drug hypersensitivity. Allergy Asthma Immunol Res. 2014; 6:156–62.

21. Na HR, Lee JM, Jung JW, Lee SY. Usefulness of drug provocation tests in children with a history of adverse drug reaction. Korean J Pediatr. 2011; 54:304–9.

22. Hur GY, Hwang EK, Moon JY, Ye YM, Shim JJ, Park HS, et al. Oral muscle relaxant may induce immediate allergic reactions. Yonsei Med J. 2012; 53:863–5.

24. Chiriac AM, Demoly P. Drug provocation tests: update and novel approaches. Allergy Asthma Clin Immunol. 2013; 9:12.

25. Demoly P, Romano A, Botelho C, Bousquet-Rouanet L, Gaeta F, Silva R, et al. Determining the negative predictive value of provocation tests with beta-lactams. Allergy. 2010; 65:327–32.

26. Blanca M, Mayorga C, Torres MJ, Reche M, Moya MC, Rodriguez JL, et al. Clinical evaluation of Pharmacia CAP System RAST FEIA amoxicil-loyl and benzylpenicilloyl in patients with penicillin allergy. Allergy. 2001; 56:862–70.
27. Sanz ML, Gamboa PM, Antépara I, Uasuf C, Vila L, Garcia-Avilés C, et al. Flow cytometric basophil activation test by detection of CD63 expression in patients with immediate-type reactions to betalactam antibiotics. Clin Exp Allergy. 2002; 32:277–86.

28. Blanca M, Romano A, Torres MJ, Férnandez J, Mayorga C, Rodriguez J, et al. Update on the evaluation of hypersensitivity reactions to betalac-tams. Allergy. 2009; 64:183–93.

29. Ayuso P, Blanca-López N, Doña I, Torres MJ, Guéant-Rodríguez RM, Canto G, et al. Advanced phenotyping in hypersensitivity drug reactions to NSAIDs. Clin Exp Allergy. 2013; 43:1097–109.

30. Pham DL, Kim JH, Trinh TH, Park HS. What we know about nonsteroidal anti-inflammatory drug hypersensitivity. Korean J Intern Med. 2016; 31:417–32.

31. Chang HS, Park JS, Jang AS, Park SW, Uh ST, Kim YH, et al. Diagnostic value of clinical parameters in the prediction of aspirin-exacerbated respiratory disease in asthma. Allergy Asthma Immunol Res. 2011; 3:256–64.

32. Bochenek G, Niżankowska-Mogilnicka E. Aspirin-exacerbated respiratory disease: clinical disease and diagnosis. Immunol Allergy Clin North Am. 2013; 33:147–61.
33. Nizankowska-Mogilnicka E, Bochenek G, Mastalerz L, Swierczyńska M, Picado C, Scadding G, et al. EAACI/GA2LEN guideline: aspirin provocation tests for diagnosis of aspirin hypersensitivity. Allergy. 2007; 62:1111–8.

34. Blanca-Lopez N, J Torres M, Doña I, Campo P, Rondón C, Seoane Reula ME, et al. Value of the clinical history in the diagnosis of urticaria/angioedema induced by NSAIDs with cross-intolerance. Clin Exp Allergy. 2013; 43:85–91.

35. Losol P, Yoo HS, Park HS. Molecular genetic mechanisms of chronic urticaria. Allergy Asthma Immunol Res. 2014; 6:13–21.

36. Knowles SR, Drucker AM, Weber EA, Shear NH. Management options for patients with aspirin and nonsteroidal antiinflammatory drug sensitivity. Ann Pharmacother. 2007; 41:1191–200.

Fig. 1.
Diagnostic flow for semisynthetic penicillin allergy. BP, benzylpenicillin; AX, amoxicillin.
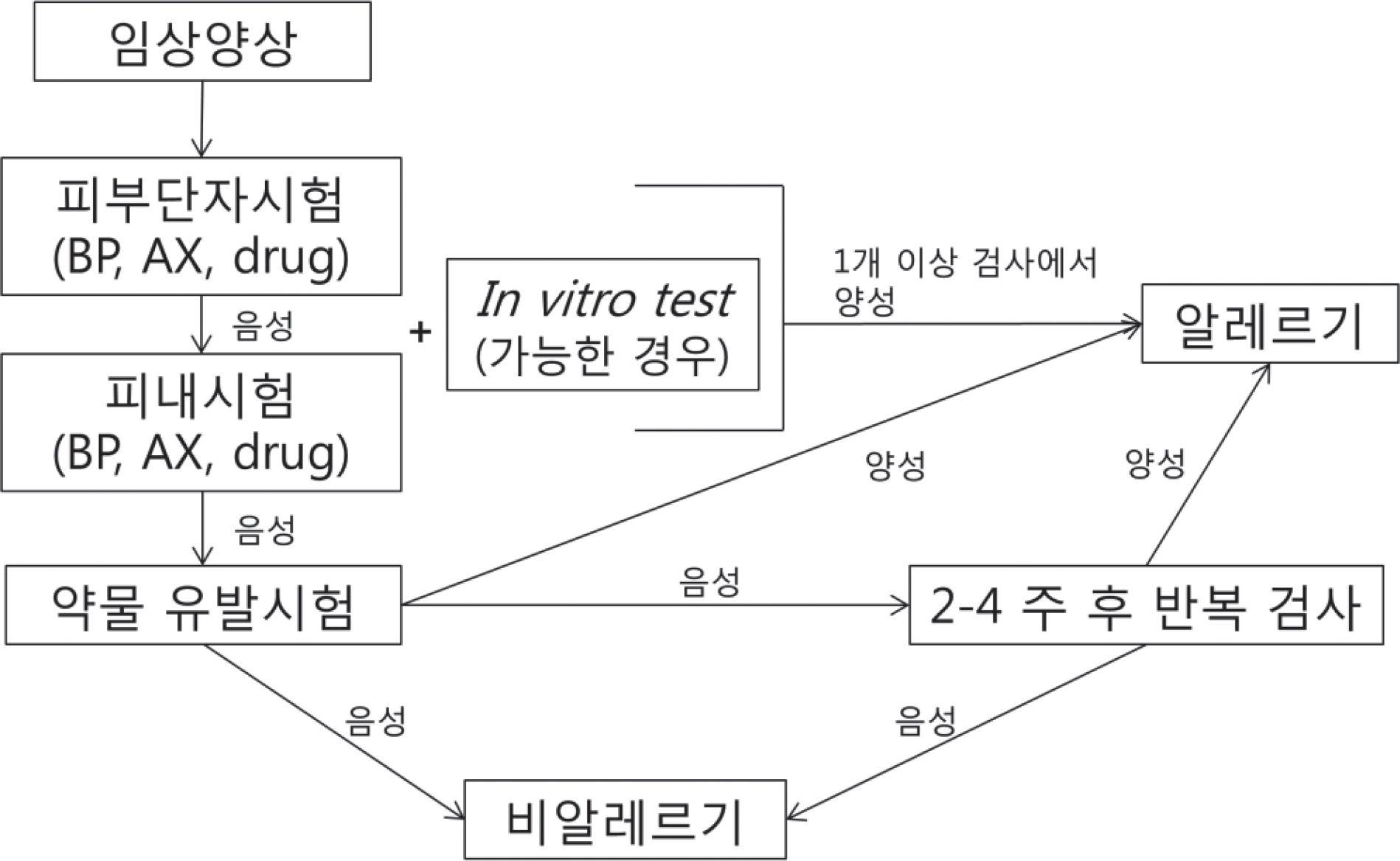
Fig. 2.
Diagnostic flow for patients with beta-lactam allergy before administering other beta-lactam antibiotics (modified from reference 8). For penicillin allergic patient before using cephalosporin (A), for cephalosporin allergic patient before using penicillin (B), and for cephalosporin allergic patient before using other cephalosporin (C).
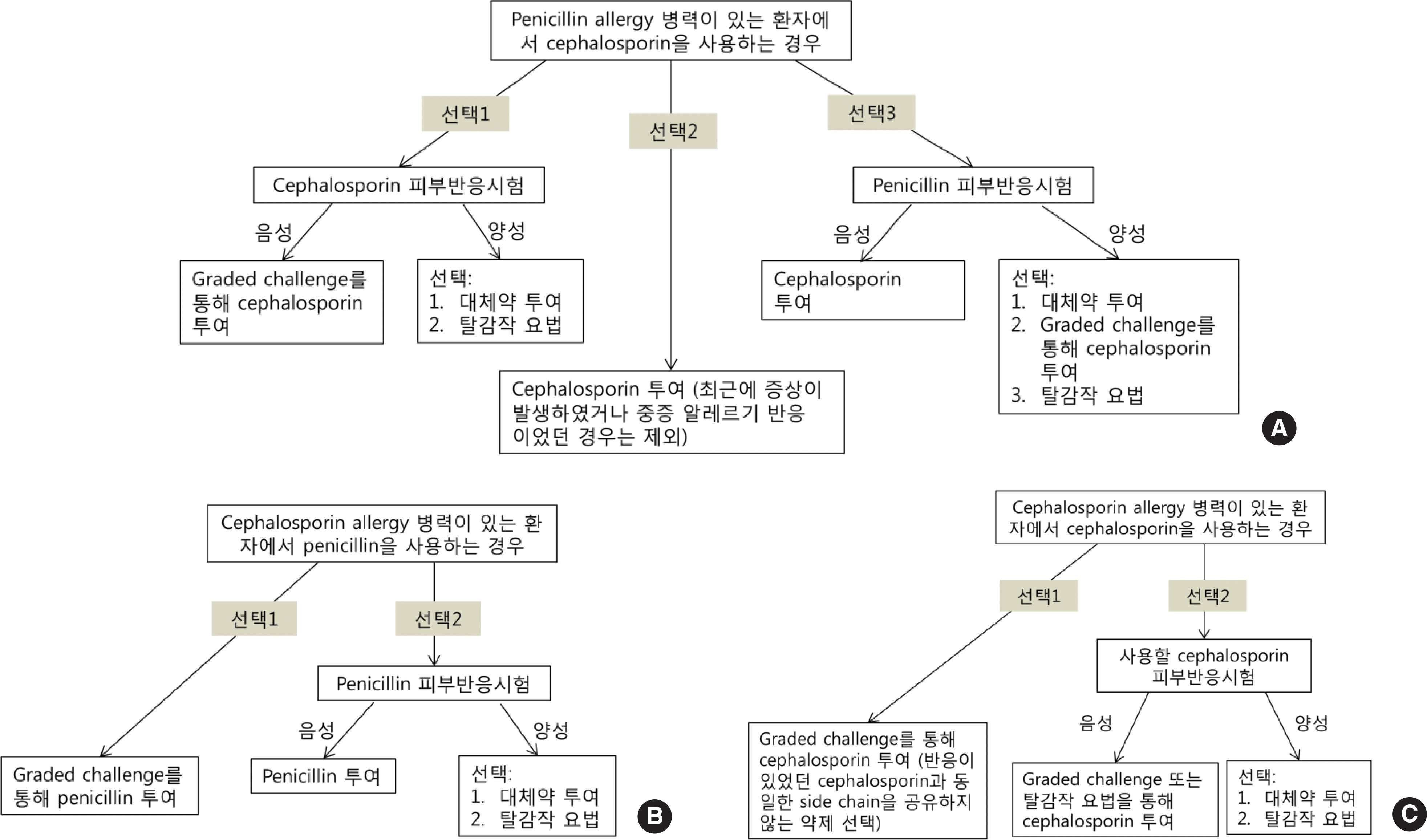
Table 1.
Drug-free interval demanded for drugs decreasing reactivity of skin tests
| Medication | Route | Immediate reaction | Nonimmediate reaction | Free interval |
|---|---|---|---|---|
| H1-antihistamines | Oral, intravenous | + | - | 5 Days |
| β-adrenergic drugs | Oral, intravenous | + | - | 5 Days |
| Glucocorticoids | Oral, intravenous | ± | - | |
| Long term | Oral, intravenous | ± | + | 3 Weeks |
| Short term, high dose | Oral, intravenous | ± | + | 1 Week |
| Short term, <50 mg∗ | Oral, intravenous | ± | - | 3 Days |
| Topical corticosteroids | Oral, intravenous | ± | + | >2 Weeks |
Table 2.
Scoring of patch test reactions




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download