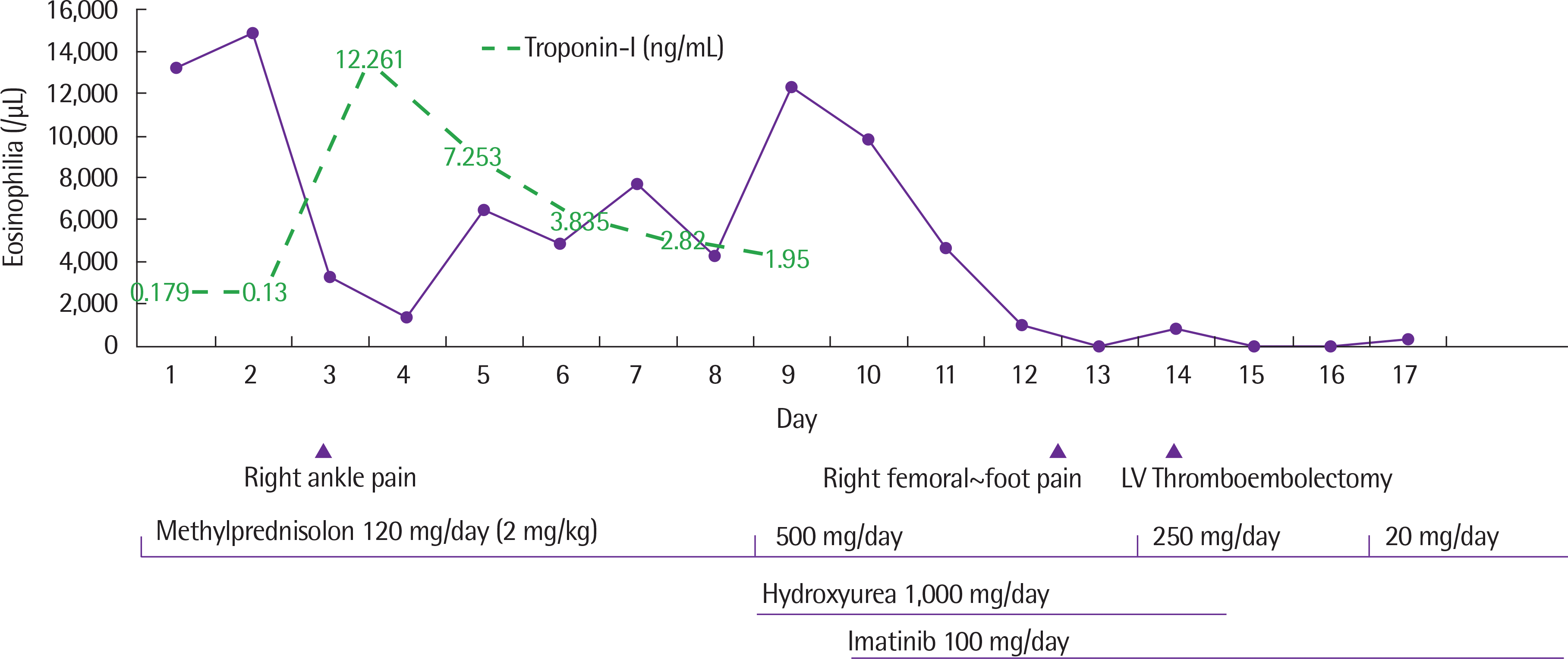
Abstract
Hypereosinophilic syndrome (HES) is a heterogeneous disorder characterized by persistent hypereosinophilia with the evidence of organ dysfunction caused by eosinophilic involvement. HES can be induced by various secondary causes, including helminthic infections, adverse drug reactions, and allergic diseases. Primary/clonal bone marrow disease, including genetic mutations in platelet driven growth factor receptor alpha (PDGFRA), platelet driven growth factor receptor beta (PDGFRB), and fibroblast growth factor receptor 1 (FGFR1) could be its causes. Although corticosteroids are the mainstay of therapy in confirmed HES, imatinib is considered a definitive treatment for HES with these mutations. However, there have been few reports about HES with these genetic mutations in Korea. Here, we report a patient who presented with sudden onset of congestive heart failure and hypereosinophilia, proved to have PDGFRB rearrangement, and was controlled successfully with imatinib after left ventricle thrombectomy.
REFERENCES
1. Haferlach T, Bacher U, Kern W, Schnittger S, Haferlach C. The diagnosis of BCR/ABL-negative chronic myeloproliferative diseases (CMPD): a comprehensive approach based on morphology, cytogenetics, and molecular markers. Ann Hematol. 2008; 87:1–10.

2. Kim TH, Gu HJ, Lee WI, Lee J, Yoon HJ, Park TS. Chronic eosinophilic leukemia with FIP1L1-PDGFRA rearrangement. Blood Res. 2016; 51:204–6.
3. Kim M, Lim J, Lee A, Park G, Kim Y, Han K, et al. A case of chronic myelomonocytic leukemia with severe eosinophilia having t(5;12)(q31;p13) with t(1;7)(q10;p10). Acta Haematol. 2005; 114:104–7.

4. Jang SE, Kang HJ, Chang YH, Lee DS, Kim HT, Koh KW, et al. A case of myeloid neoplasm with the PDGFRB rearrangement and eosinophilia. Korean J Med. 2010; 78:386–90.
5. Shin SY, Jung CW, Choi DC, Lee BJ, Kim HJ, Kim SH. Chronic eosinophilic leukemia with a FIP1L1-PDGFRA rearrangement: Two case reports and a review of Korean cases. Blood Res. 2015; 50:58–61.
6. Keene P, Mendelow B, Pinto MR, Bezwoda W, MacDougall L, Falkson G, et al. Abnormalities of chromosome 12p13 and malignant proliferation of eosinophils: a nonrandom association. Br J Haematol. 1987; 67:25–31.
7. Golub TR, Barker GF, Lovett M, Gilliland DG. Fusion of PDGF receptor beta to a novel ets-like gene, tel, in chronic myelomonocytic leukemia with t(5;12) chromosomal translocation. Cell. 1994; 77:307–16.
8. Bain BJ, Fletcher SH. Chronic eosinophilic leukemias and the myeloproliferative variant of the hypereosinophilic syndrome. Immunol Allergy Clin North Am. 2007; 27:377–88.

9. Walz C, Metzgeroth G, Haferlach C, Schmitt-Graeff A, Fabarius A, Ha-gen V, et al. Characterization of three new imatinib-responsive fusion genes in chronic myeloproliferative disorders generated by disruption of the platelet-derived growth factor receptor beta gene. Haematologica. 2007; 92:163–9.
10. Wittman B, Horan J, Baxter J, Goldberg J, Felgar R, Baylor E, et al. A 2-year-old with atypical CML with a t(5;12)(q33;p13) treated successfully with imatinib mesylate. Leuk Res. 2004; 28(Suppl 1):S65–9.

11. Cools J, DeAngelo DJ, Gotlib J, Stover EH, Legare RD, Cortes J, et al. A tyrosine kinase created by fusion of the PDGFRA and FIP1L1 genes as a therapeutic target of imatinib in idiopathic hypereosinophilic syndrome. N Engl J Med. 2003; 348:1201–14.
12. David M, Cross NC, Burgstaller S, Chase A, Curtis C, Dang R, et al. Du-rable responses to imatinib in patients with PDGFRB fusion gene-positive and BCR-ABL-negative chronic myeloproliferative disorders. Blood. 2007; 109:61–4.

13. Jovanovic JV, Score J, Waghorn K, Cilloni D, Gottardi E, Metzgeroth G, et al. Low-dose imatinib mesylate leads to rapid induction of major molecular responses and achievement of complete molecular remission in FIP1L1-PDGFRA-positive chronic eosinophilic leukemia. Blood. 2007; 109:4635–40.

14. Klion AD, Robyn J, Maric I, Fu W, Schmid L, Lemery S, et al. Relapse following discontinuation of imatinib mesylate therapy for FIP1L1/PDG-FRA-positive chronic eosinophilic leukemia: implications for optimal dosing. Blood. 2007; 110:3552–6.

15. Ogbogu PU, Bochner BS, Butterfield JH, Gleich GJ, Huss-Marp J, Kahn JE, et al. Hypereosinophilic syndrome: a multicenter, retrospective analysis of clinical characteristics and response to therapy. J Allergy Clin Immunol. 2009; 124:1319–25.e3.

17. Pitini V, Arrigo C, Azzarello D, La Gattuta G, Amata C, Righi M, et al. Serum concentration of cardiac Troponin T in patients with hypereosinophilic syndrome treated with imatinib is predictive of adverse outcomes. Blood. 2003; 102:3456–7.

18. Chusid MJ, Dale DC, West BC, Wolff SM. The hypereosinophilic syndrome: analysis of fourteen cases with review of the literature. Medicine (Baltimore). 1975; 54:1–27.
19. Lefebvre C, Bletry O, Degoulet P, Guillevin L, Bentata-Pessayre M, Le Thi Huong Du, et al. Prognostic factors of hypereosinophilic syndrome. Study of 40 cases. Ann Med Interne (Paris). 1989; 140:253–7.
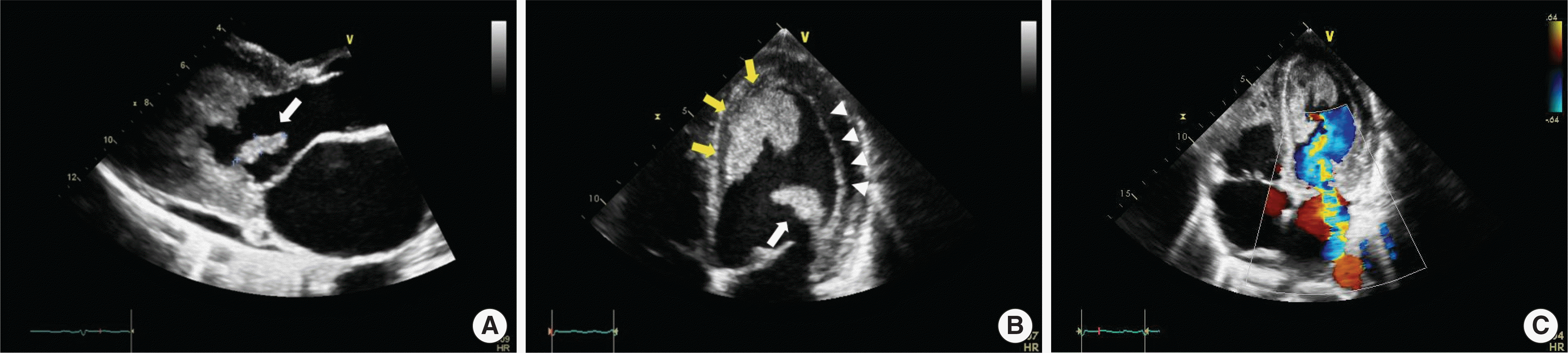
Fig. 1.
Transthoracic echocardiography image showing cardiac involvement of hypereosinophilia. (A) Parasternal long-axis view demonstrates a hyperechoic mobile mass (2.0×0.6-cm size) suggesting thrombus (white arrow) at the postero-lateral myocardial wall. (B) Apical 4-chamber view demonstrates a large thrombus filling the left ventricular cavity (yellow arrows) and a hyper-mobile thrombus (white arrow) at the myocardial wall. This view also shows the endomyocardial fibrosis extend-ing along lateral wall of the ventricle (arrowheads). (C) The posterior mitral valve leaflet has suffered tissue injury and it has developed moderate to severe eccentric mitral regurgitation (color doppler).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download