Abstract
Purpose
Allergic rhinitis is one of the most common chronic diseases that affect in sleep, fatigue, headache, impaired cognition, and performances at work or school. Monitoring rhinitis control is important, because rhinitis is a life-long disease and affects patients’ health-related quality of life. The rhinitis control assessment test (RCAT) completed its development and initial validation, following confirmation of its reliability, validity, and responsiveness in the United States. To apply the RCAT in Korean clinical practice, we conducted linguistic adaptation of the RCAT in Korean language.
Methods
The process of linguistic adaptation was composed of 10 steps: preparation, forward translation, reconciliation, back translation, back translation review, harmonization, cognitive debriefing, review of cognitive debriefing results and finalization, proofreading, and the final report.
Results
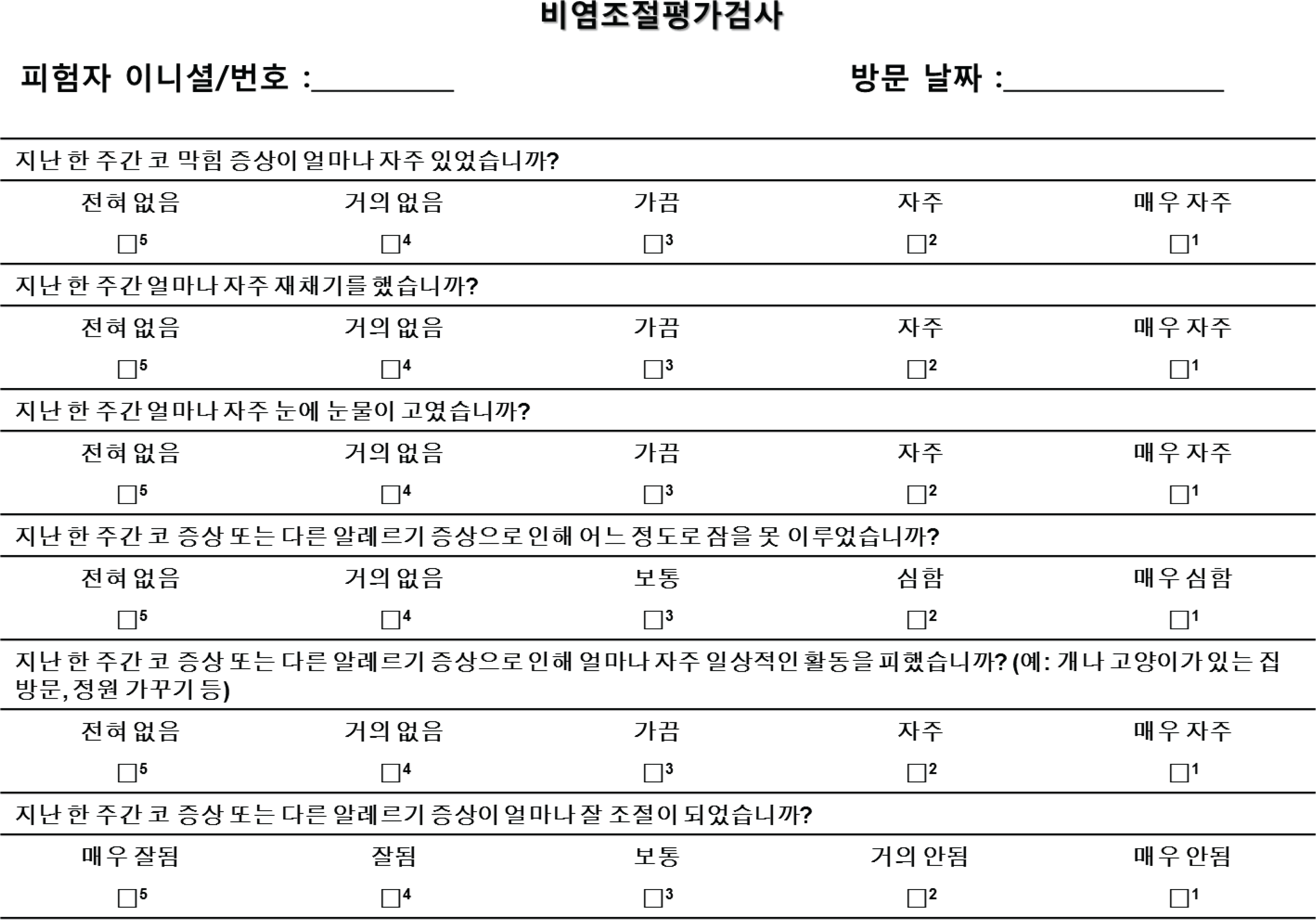
We completed a Korean version of the RCAT according to 10 steps. The Korean version of the RCAT was composed of 6 items, including nasal and ocular symptoms, sleep disturbances, limitation of casual activity, and symptom control. The score ranged from 5 to 30. Higher score indicated the well-controlled status of rhinitis.
Go to : 
REFERENCES
1. Rhee CS, Wee JH, Ahn JC, Lee WH, Tan KL, Ahn S, et al. Prevalence, risk factors and comorbidities of allergic rhinitis in South Korea: The Fifth Korea National Health and Nutrition Examination Survey. Am J Rhinol Allergy. 2014; 28:e107–14.

2. Yoo KH, Ahn HR, Park JK, Kim JW, Nam GH, Hong SK, et al. Burden of Respiratory Disease in Korea: An Observational Study on Allergic Rhinitis, Asthma, COPD, and Rhinosinusitis. Allergy Asthma Immunol Res. 2016; 8:527–34.

3. Kim Y, Seo JH, Kwon JW, Lee E, Yang SI, Cho HJ, et al. The prevalence and risk factors of allergic rhinitis from a nationwide study of Korean elementary, middle, and high school students. Allergy Asthma Respir Dis. 2015; 3:272–80.

4. Brozek JL, Bousquet J, Baena-Cagnani CE, Bonini S, Canonica GW, Ca-sale TB, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines: 2010 revision. J Allergy Clin Immunol. 2010; 126:466–76.
5. Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy. 2008; 63(Suppl 86):8–160.
6. Global Initiative for Asthma. 2017 GINA report, global strategy for asthma management and prevention [Internet]. Global Initiative for Asthma;2016. [cited 2017 Jan 8]. Available from. http://ginasthma.org/2017-gina-report-global-strategy-for-asthma-management-and-prevention/.
7. Nathan RA, Dalal AA, Stanford RH, Meltzer EO, Schatz M, Derebery J, et al. Qualitative Development of the Rhinitis Control Assessment Test (RCAT), an Instrument for Evaluating Rhinitis Symptom Control. Patient. 2010; 3:91–9.

8. Schatz M, Meltzer EO, Nathan R, Derebery MJ, Mintz M, Stanford RH, et al. Psychometric validation of the rhinitis control assessment test: a brief patient-completed instrument for evaluating rhinitis symptom control. Ann Allergy Asthma Immunol. 2010; 104:118–24.

9. Meltzer EO, Schatz M, Nathan R, Garris C, Stanford RH, Kosinski M. Reliability, validity, and responsiveness of the Rhinitis Control Assessment Test in patients with rhinitis. J Allergy Clin Immunol. 2013; 131:379–86.

10. Wild D, Grove A, Martin M, Eremenco S, McElroy S, Verjee-Lorenz A, et al. Principles of good practice for the translation and cultural adaptation process for patient-reported outcomes (PRO) measures: report of the ISPOR Task Force for Translation and Cultural Adaptation. Value Health. 2005; 8:94–104.

11. Nathan RA. The rhinitis control assessment test: implications for the present and future. Curr Opin Allergy Clin Immunol. 2014; 14:13–9.
12. Fernandes PH, Matsumoto F, Solé D, Wandalsen GF. Translation into Portuguese and validation of the Rhinitis Control Assessment Test (RCAT) questionnaire. Braz J Otorhinolaryngol. 2016; 82:674–9.

13. Lee E, Kim MJ, Yang SI, Yu J, Hong SJ. Comparison of short-term effects between subcutaneous and sublingual immunotherapies in children with house dust mite-sensitized allergic rhinitis and asthma. Allergy Asthma Respir Dis. 2015; 3:180–6.

14. Kang HY, Moon SH, Jang HJ, Lim DH, Kim JH. Validation of “ quality-of-life questionnaire in Korean children with allergic rhinitis” in middle school students. Allergy Asthma Respir Dis. 2016; 4:369–73.
15. Juniper EF, Thompson AK, Ferrie PJ, Roberts JN. Validation of the standardized version of the Rhinoconjunctivitis Quality of Life Questionnaire. J Allergy Clin Immunol. 1999; 104(2 Pt 1):364–9.

16. Park KH, Cho JS, Lee KH, Shin SY, Moon JH, Cha CI. Rhinoconjunctivitis quality of life questionnaire (RQLQ) as an evaluator of perennial allergic rhinitis patients-the first report-. Korean J Otolaryngol-Head Neck Surg. 2002; 45:254–62.
Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download