Abstract
Purpose
Atopic dermatitis is often accompanied by food allergies which occur through skin barrier defects. Especially Staphylococcus aureus colonization can exacerbate skin barrier defects that cause sensitization and increase specific IgE (sIgE) to food. We investigated the association between skin colonization and food sIgE changes in children with atopic dermatitis.
Methods
Atopic dermatitis was diagnosed by a pediatric allergist in patients between 3 months and 3 years of age. Total IgE and sIgE to egg white, cow's milk, wheat, and peanuts were taken. Eosinophil count and eosinophil cationic protein were also taken. Comparisons were done between the groups with and without S. aureus colonization.
Results
It was found that 50.3% of the 294 enrolled patients had S. aureus colonization on lesional skin. Statistically significant sensitization to wheat and peanut were increased with S. aureus colonization. Statistically significant increases in sIgE (above cutoff level) were also found in egg white, milk, wheat and peanut. Higher S. aureus colony counts also increased sIgE of all foods. Methicillin-re-sistant S. aureus showed no statistical difference compared to methicillin-susceptible S. aureus in severity and sIgE levels.
Go to : 
REFERENCES
1. Flohr C, Mann J. New insights into the epidemiology of childhood atopic dermatitis. Allergy. 2014; 69:3–16.

2. Boyce JA, Assa'ad A, Burks AW, Jones SM, Sampson HA, Wood RA, et al. Guidelines for the diagnosis and management of food allergy in the United States: summary of the NIAID-Sponsored Expert Panel Report. J Allergy Clin Immunol. 2010; 126:1105–18.

3. Werfel T, Breuer K. Role of food allergy in atopic dermatitis. Curr Opin Allergy Clin Immunol. 2004; 4:379–85.

4. Leung DY, Guttman-Yassky E. Deciphering the complexities of atopic dermatitis: shifting paradigms in treatment approaches. J Allergy Clin Immunol. 2014; 134:769–79.

6. McFadden JP, Noble WC, Camp RD. Superantigenic exotoxin-secreting potential of staphylococci isolated from atopic eczematous skin. Br J Dermatol. 1993; 128:631–2.

7. Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol (Stockh). 1980; 92:44–7.
8. Sampson HA. Utility of food-specific IgE concentrations in predicting symptomatic food allergy. J Allergy Clin Immunol. 2001; 107:891–6.

9. Goodyear HM, Watson PJ, Egan SA, Price EH, Kenny PA, Harper JI. Skin microflora of atopic eczema in first time hospital attenders. Clin Exp Dermatol. 1993; 18:300–4.

10. Lipnharski C, d'Azevedo PA, Quinto VP, Bessa G, Bonamigo RR. Colonization by S. aureus increases the EASI and the number of appointments by patients with atopic dermatitis: cohort with 93 patients. An Bras Dermatol. 2013; 88:518–21.
11. Jagadeesan S, Kurien G, Divakaran MV, Sadanandan SM, Sobhanaku-mari K, Sarin A. Methicillin-resistant Staphylococcus aureus colonization and disease severity in atopic dermatitis: a cross-sectional study from South India. Indian J Dermatol Venereol Leprol. 2014; 80:229–34.

12. Hon KL, Tsang YC, Pong NH, Ng C, Ip M, Leung TF. Clinical features and Staphylococcus aureus colonization/infection in childhood atopic dermatitis. J Dermatolog Treat. 2016; 27:235–40.
13. Kobayashi T, Glatz M, Horiuchi K, Kawasaki H, Akiyama H, Kaplan DH, et al. Dysbiosis and Staphylococcus aureus colonization drives inflammation in atopic dermatitis. Immunity. 2015; 42:756–66.

14. Jones AL, Curran-Everett D, Leung DY. Food allergy is associated with Staphylococcus aureus colonization in children with atopic dermatitis. J Allergy Clin Immunol. 2016; 137:1247–8.e1-3.

16. Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002; 347:1151–60.

17. Schlievert PM, Strandberg KL, Lin YC, Peterson ML, Leung DY. Secreted virulence factor comparison between methicillin-resistant and methicil-lin-sensitive Staphylococcus aureus, and its relevance to atopic dermatitis. J Allergy Clin Immunol. 2010; 125:39–49.

18. Strange P, Skov L, Lisby S, Nielsen PL, Baadsgaard O. Staphylococcal enterotoxin B applied on intact normal and intact atopic skin induces dermatitis. Arch Dermatol. 1996; 132:27–33.

19. Ganeshan K, Neilsen CV, Hadsaitong A, Schleimer RP, Luo X, Bryce PJ. Impairing oral tolerance promotes allergy and anaphylaxis: a new murine food allergy model. J Allergy Clin Immunol. 2009; 123:231–8.e4.

20. Neuber K, Steinrücke K, Ring J. Staphylococcal enterotoxin B affects in vitro IgE synthesis, interferon-gamma, interleukin-4 and interleukin-5 production in atopic eczema. Int Arch Allergy Immunol. 1995; 107:179–82.
21. Forbes EE, Camberis M, Prout M, Tang S, Paul WE, Le Gros G. Bacterial superantigen elicits food-induced allergic sensitization through epicuta-neous exposure. J Allergy Clin Immunol. 2010; 125(2 Suppl 1):AB113.

22. Hauk PJ, Hamid QA, Chrousos GP, Leung DY. Induction of corticosteroid insensitivity in human PBMCs by microbial superantigens. J Allergy Clin Immunol. 2000; 105:782–7.

23. Komata T, Söderström L, Borres MP, Tachimoto H, Ebisawa M. The predictive relationship of food-specific serum IgE concentrations to challenge outcomes for egg and milk varies by patient age. J Allergy Clin Immunol. 2007; 119:1272–4.

Go to : 
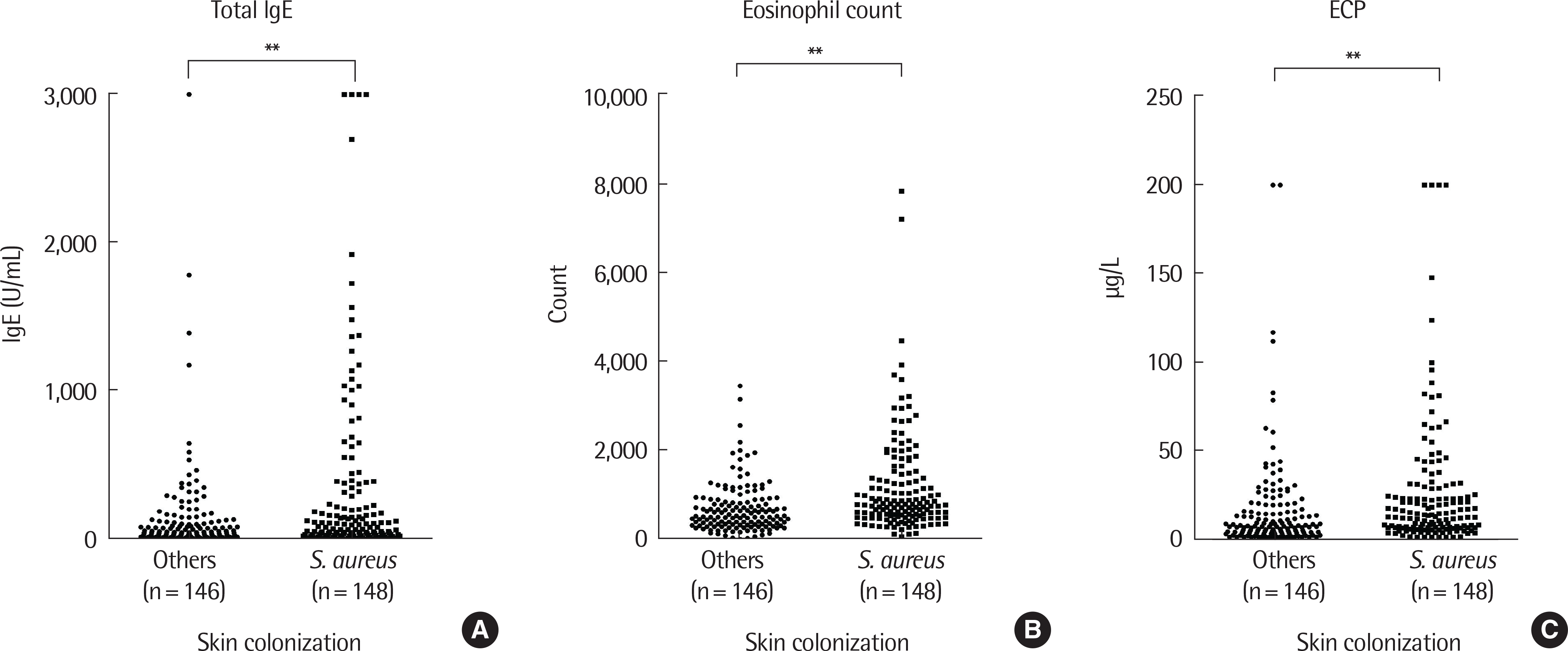 | Fig. 1.Comparisons of total IgE (A), eosinophil count (B), and eosinophil cationic protein (ECP) (C) were done between the groups with and without Staphylococus aureus colonization. S. aureus colonization showed statistically significant (P<0.05) increases in total IgE, eosinophil count, and ECP compared to the group without colonization. ∗∗ P<0.01. |
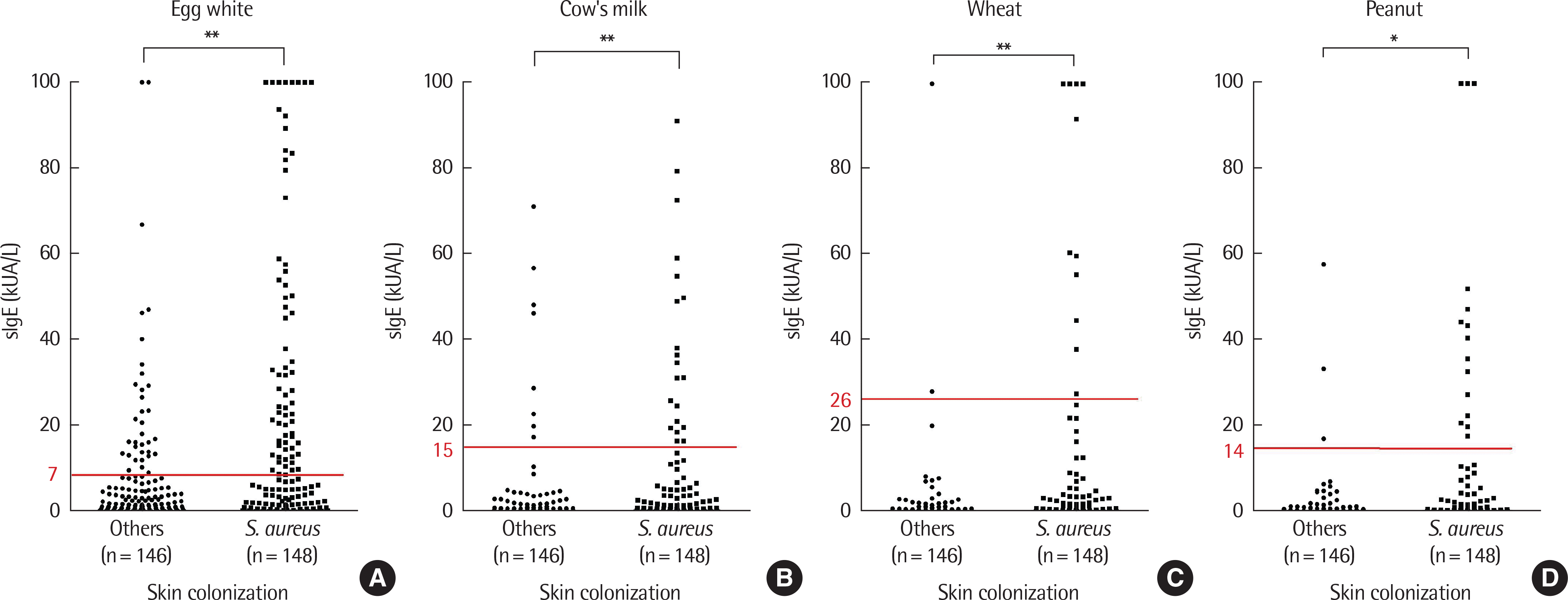 | Fig. 2.Comparisons of specific IgE (slgE) (egg white [A], cow's milk [B], wheat [C], and peanuts [D]) were done between the groups with and without Staphylococus aureus colonization. Statistically significant (P<0.05) differences were showed in all slgE between 2 groups. ∗ P<0.05. ∗∗ P<0.01. |
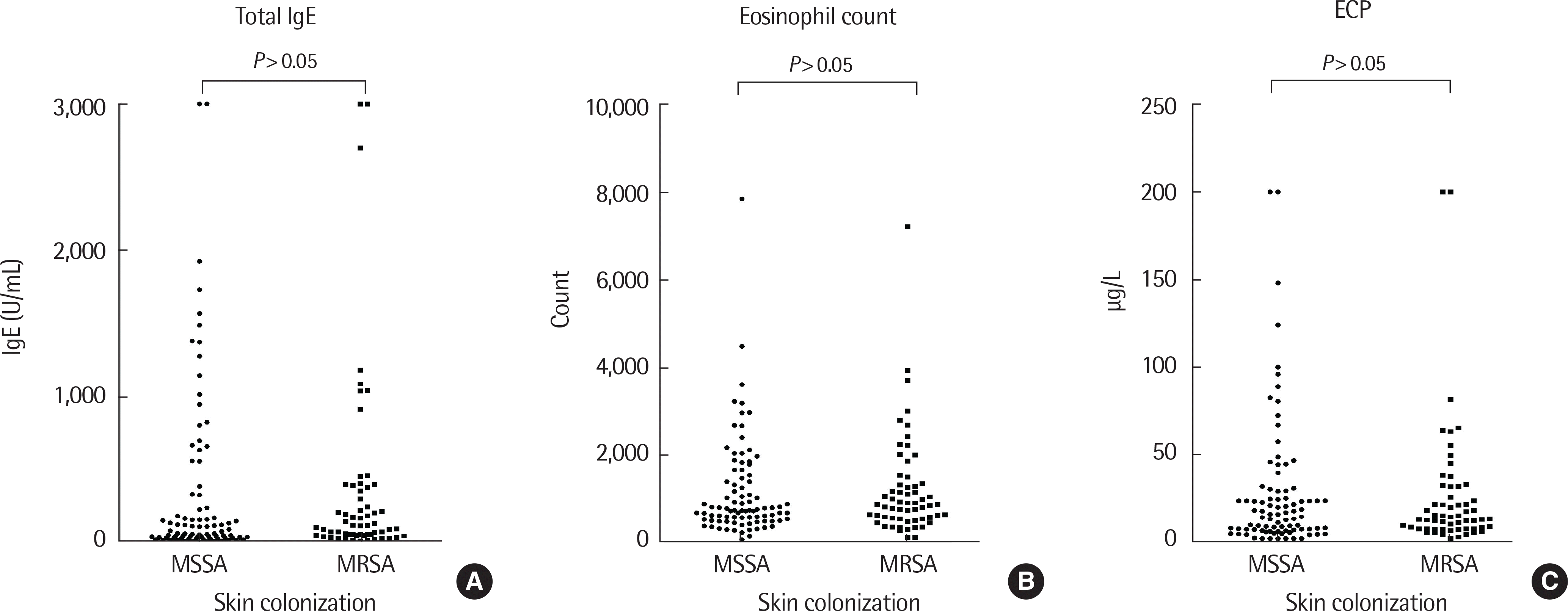 | Fig. 3.Comparisons of total IgE (A), eosinophil count (B), and cationic protein (ECP) (C) were done between the groups with and without sensitization to methicillin. Statistically significant (P<0.05) differences in total IgE, eosinophil count, and ECP were not found between the methicillin resistant and nonresistant groups. MSSA, methicillin-susceptible Staphylococcus aureus; MRSA, methichillin-resistant Staphylococcus aureus. |
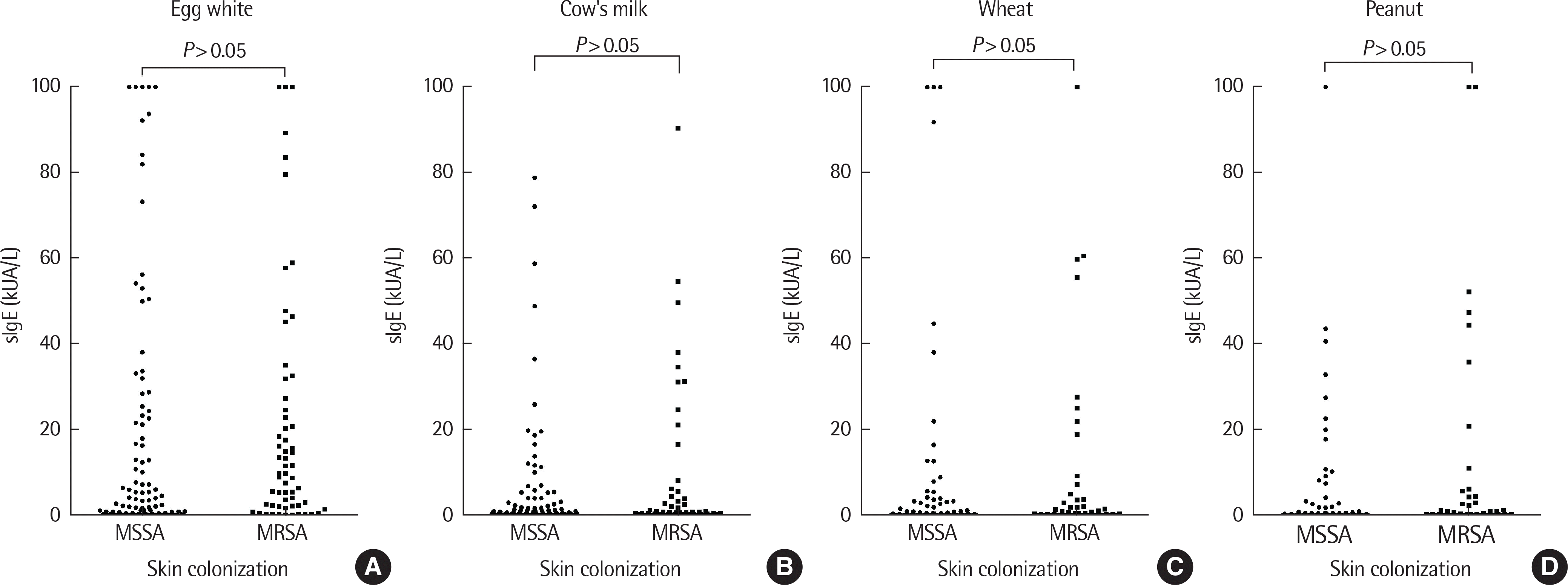 | Fig. 4.Comparisons of specific IgE (slgE) were done between methicillin resistant and nonresistant groups. The results were not statistically significant according to each slgE. Methicillin resistant versus nonresistant slgE results were egg white (A), milk (B), wheat (C), and peanuts (D), respectively. The resistant group showed higher values but were not statistically significant (P>0.05). MSSA, methicillin-susceptible Staphylococcus aureus; MRSA, methichillin-resistant Staphylococcus aureus. |
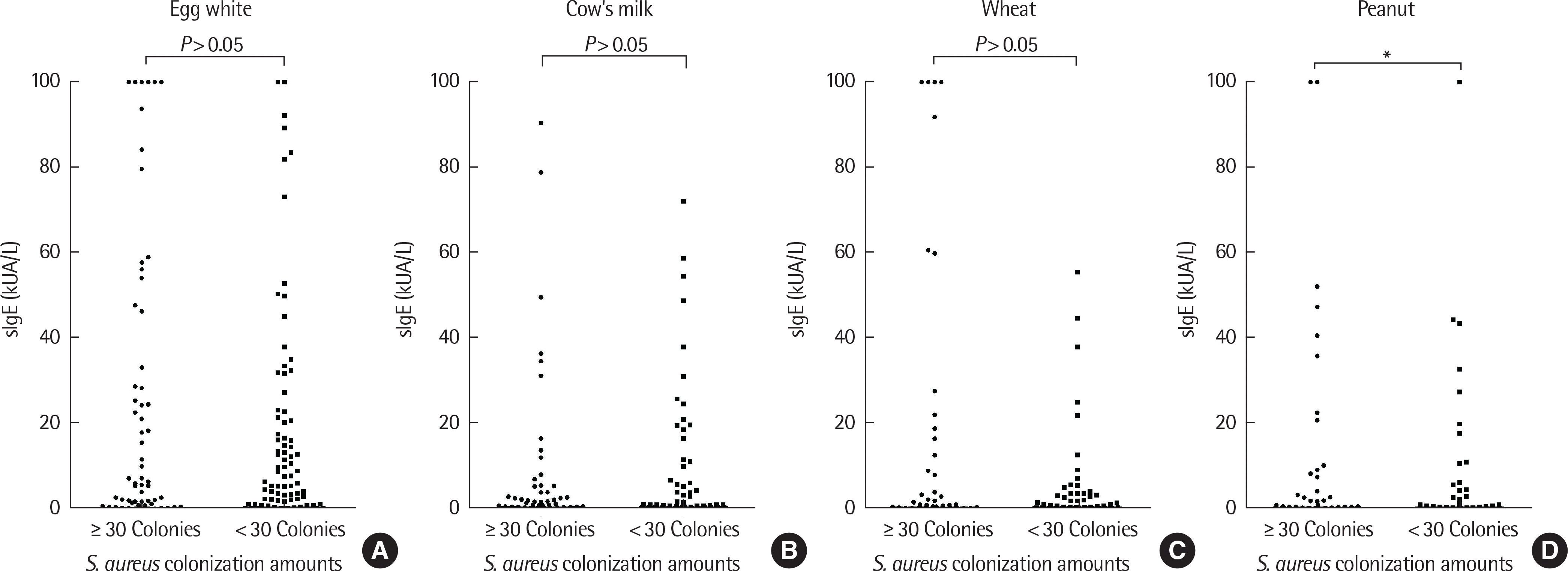 | Fig. 5.We investigated the association between Staphylococus aureus colony counts and specific lgE (slgE). Statistically significant increments in slgE for peanuts were found with proportional increase in S. aureus colony count. More than 30 colonies vs. less than 30 colonies slgE were egg white (A), milk (B), wheat (C), and peanuts (D), respectively. ∗ P<0.05. |
Table 1.
Dermographic characteristics of the children with atopic dermatitis (n=294)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download