Abstract
Community-acquired pneumonia is the leading cause of pediatric morbidity and mortality. However, there is a lack of data on the epidemiology of pneumonia in Korean children. In this review, we aimed to summarize pneumonia studies in Korea and suggest diagnostic methods and treatment for Korean children who were referred based on the foreign guidelines for pediatric community-acquired pneumonia. A Korean guideline for pediatric pneumonia in tune with domestic circumstances is needed.
REFERENCES
1. Walker CL, Rudan I, Liu L, Nair H, Theodoratou E, Bhutta ZA, et al. Global burden of childhood pneumonia and diarrhoea. Lancet. 2013; 381:1405–16.

3. McCabe C, Kirchner C, Zhang H, Daley J, Fisman DN. Guideline-con-cordant therapy and reduced mortality and length of stay in adults with community-acquired pneumonia: playing by the rules. Arch Intern Med. 2009; 169:1525–31.
4. Bradley JS, Byington CL, Shah SS, Alverson B, Carter ER, Harrison C, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011; 53:e25–76.

5. Lee CH, Won YK, Roh EJ, Suh DI, Chung EH. A nationwide study of children and adolescents with pneumonia who visited Emergency Department in South Korea in 2012. Korean J Pediatr. 2016; 59:132–8.

6. Lee YH, Shin YL, Suh WS, Shin MY, Park JO. A clinical study of lobar/lobular pneumonia in children. Pediatr Allergy Respir Dis. 2009; 19:271–81.
7. Park KM, Son SK, Kim HY, Kim YW, Hwang JY, Park HJ. Clinical features of necrotizing pneumonia in children. Allergy Asthma Respir Dis. 2014; 2:208–12.

8. Cheong HY, Lee JH, Kim YB, Nam HS, Choi YJ, Kim CJ, et al. Viral etiologic agents in acute viral lower respiratory tract detected by Multiplex RT-PCR. Pediatr Allergy Respir Dis (Korea). 2007; 17:344–53.
9. Moon JH, Suh KJ, Chung EH, Shin MY, Lee JS, Park YM, et al. Epidemiology of acute viral lower respiratory tract infection in hospitalized children in two different areas of Korea. Korean J Pediatr Infect Dis. 2002; 9:193–200.

10. Kim HY, Kim KM, Kim SH, Son SK, Park HJ. Clinical manifestations of respiratory viruses in hospitalized children with acute viral lower respiratory tract infections from 2010 to 2011 in Busan and Gyeongsangnam-do, Korea. Pediatr Allergy Respir Dis. 2012; 22:265–72.

11. Kwon JH, Chung YH, Lee NY, Chung EH, Ahn KM, Lee SI. An epidemiological study of acute viral lower respiratory tract infections in hospitalized children from 2002 to 2006 in Seoul, Korea. Pediatr Allergy Respir Dis. 2008; 18:26–36.
12. Kim EK, Youn YS, Rhim JW, Shin MS, Kang JH, Lee KY. Epidemiological comparison of three Mycoplasma pneumoniae pneumonia epidemics in a single hospital over 10 years. Korean J Pediatr. 2015; 58:172–7.
13. Ahn YH, Park SH. Clinical considerations about Mycoplasma pneumoniae pneumonia in the young, between 2003 and 2006. Pediatr Allergy Respir Dis. 2007; 17:249–59.
14. Yang EA, Gang MH, You SY, Kim JH, Lee JH. Clinical characteristics of children with lobar pneumonia caused by Mycoplasma pneumoniae. Pediatr Allergy Respir Dis. 2012; 22:256–64.
15. Hong KB, Choi EH, Lee HJ, Lee SY, Cho EY, Choi JH, et al. Macrolide resistance of Mycoplasma pneumoniae, South Korea, 2000-2011. Emerg Infect Dis. 2013; 19:1281–4.
16. Lee KY, Lee HS, Hong JH, Lee MH, Lee JS, Burgner D, et al. Role of prednisolone treatment in severe Mycoplasma pneumoniae pneumonia in children. Pediatr Pulmonol. 2006; 41:263–8.
17. Yang J, Hooper WC, Phillips DJ, Talkington DF. Cytokines in Mycoplasma pneumoniae infections. Cytokine Growth Factor Rev. 2004; 15:157–68.

18. You SY, Jwa HJ, Yang EA, Kil HR, Lee JH. Effects of methylprednisolone pulse therapy on refractory Mycoplasma pneumoniae pneumonia in children. Allergy Asthma Immunol Res. 2014; 6:22–6.
19. Youn YS, Lee SC, Rhim JW, Shin MS, Kang JH, Lee KY. Early additional immune-modulators for Mycoplasma pneumoniae pneumonia in children: An Observation Study. Infect Chemother. 2014; 46:239–47.
20. Advani S, Sengupta A, Forman M, Valsamakis A, Milstone AM. Detecting respiratory viruses in asymptomatic children. Pediatr Infect Dis J. 2012; 31:1221–6.

21. Song JH, Lee NY, Ichiyama S, Yoshida R, Hirakata Y, Fu W, et al. Spread of drug-resistant Streptococcus pneumoniae in Asian countries: Asian Network for Surveillance of Resistant Pathogens (ANSORP) Study. Clin Infect Dis. 1999; 28:1206–11.
22. Baquero F. Pneumococcal resistance to beta-lactam antibiotics: a global geographic overview. Microb Drug Resist. 1995; 1:115–20.
23. Kim SH, Song JH, Chung DR, Thamlikitkul V, Yang Y, Wang H, et al. Changing trends in antimicrobial resistance and serotypes of Streptococcus pneumoniae isolates in Asian countries: an Asian Network for Surveillance of Resistant Pathogens (ANSORP) study. Antimicrob Agents Chemother. 2012; 56:1418–26.

24. Newman RE, Hedican EB, Herigon JC, Williams DD, Williams AR, New-land JG. Impact of a guideline on management of children hospitalized with community-acquired pneumonia. Pediatrics. 2012; 129:e597–604.

25. Williams DJ, Edwards KM, Self WH, Zhu Y, Ampofo K, Pavia AT, et al. Antibiotic choice for children hospitalized with pneumonia and adherence to national guidelines. Pediatrics. 2015; 136:44–52.

26. Song JH, Jung SI, Ko KS, Kim NY, Son JS, Chang HH, et al. High prevalence of antimicrobial resistance among clinical Streptococcus pneumoniae isolates in Asia (an ANSORP study). Antimicrob Agents Chemother. 2004; 48:2101–7.
27. Hendaus MA, Jomha FA, Alhammadi AH. Virus-induced secondary bacterial infection: a concise review. Ther Clin Risk Manag. 2015; 11:1265–71.

28. Beadling C, Slifka MK. How do viral infections predispose patients to bacterial infections? Curr Opin Infect Dis. 2004; 17:185–91.

29. Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis. 2008; 198:962–70.

30. Deng JC. Viral-bacterial interactions-therapeutic implications. Influenza Other Respir Viruses. 2013; 7(Suppl 3):24–35.

31. Lucero MG, Dulalia VE, Nillos LT, Williams G, Parreño RA, Nohynek H, et al. Pneumococcal conjugate vaccines for preventing vaccine-type invasive pneumococcal disease and X-ray defined pneumonia in children less than two years of age. Cochrane Database Syst Rev. 2009; (4):CD004977.

32. de St Maurice A, Grijalva CG, Fonnesbeck C, Schaffner W, Halasa NB. Racial and regional differences in rates of invasive pneumococcal disease. Pediatrics. 2015; 136:e1186–94.

33. Kronman MP, Hersh AL, Feng R, Huang YS, Lee GE, Shah SS. Ambula-tory visit rates and antibiotic prescribing for children with pneumonia, 1994-2007. Pediatrics. 2011; 127:411–8.

34. Shaughnessy EE, Stalets EL, Shah SS. Community-acquired pneumonia in the post 13-valent pneumococcal conjugate vaccine era. Curr Opin Pediatr. 2016; 28:786–93.

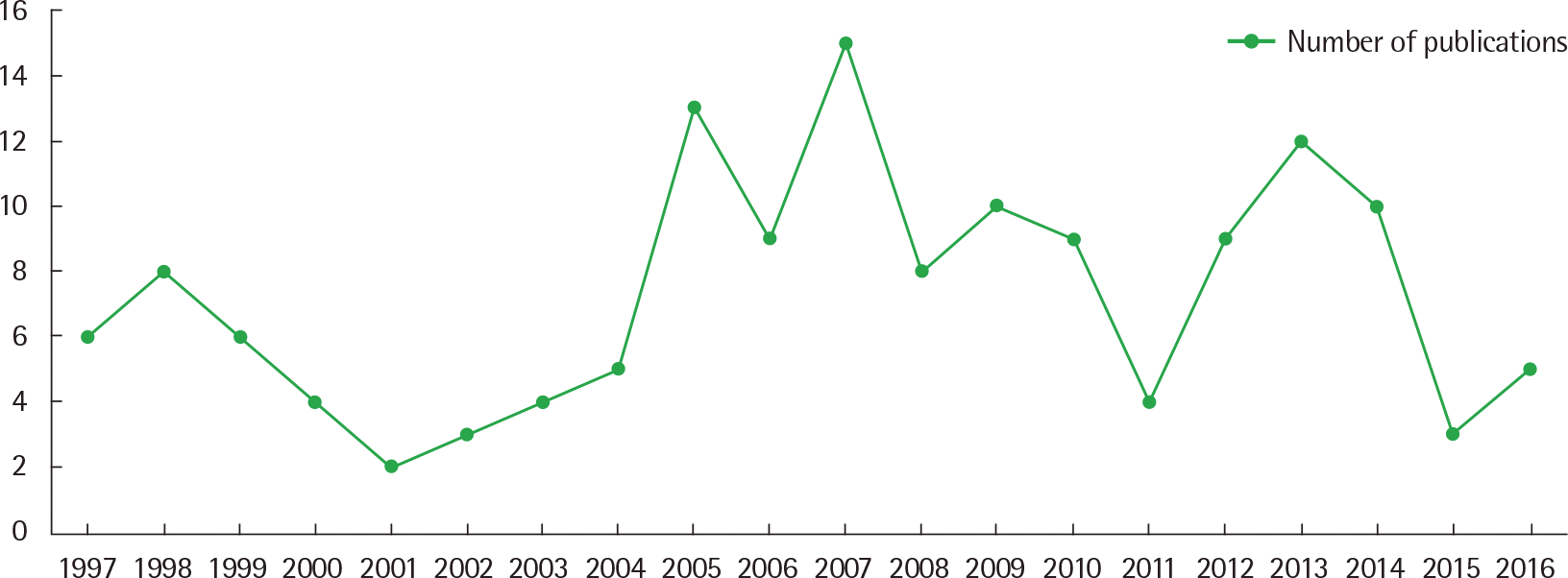
Fig. 2.
(A) Number of publications on pneumonia in Korean children according to Korea Citation Index (KCI) or Science Citation Index (Expanded) (SCI(E)), (B) Number of papers about pneumonia in Korean children published by Allergy, Asthma and Respirator Diseases or Pediatric Infection and Vaccine during past 20 years.
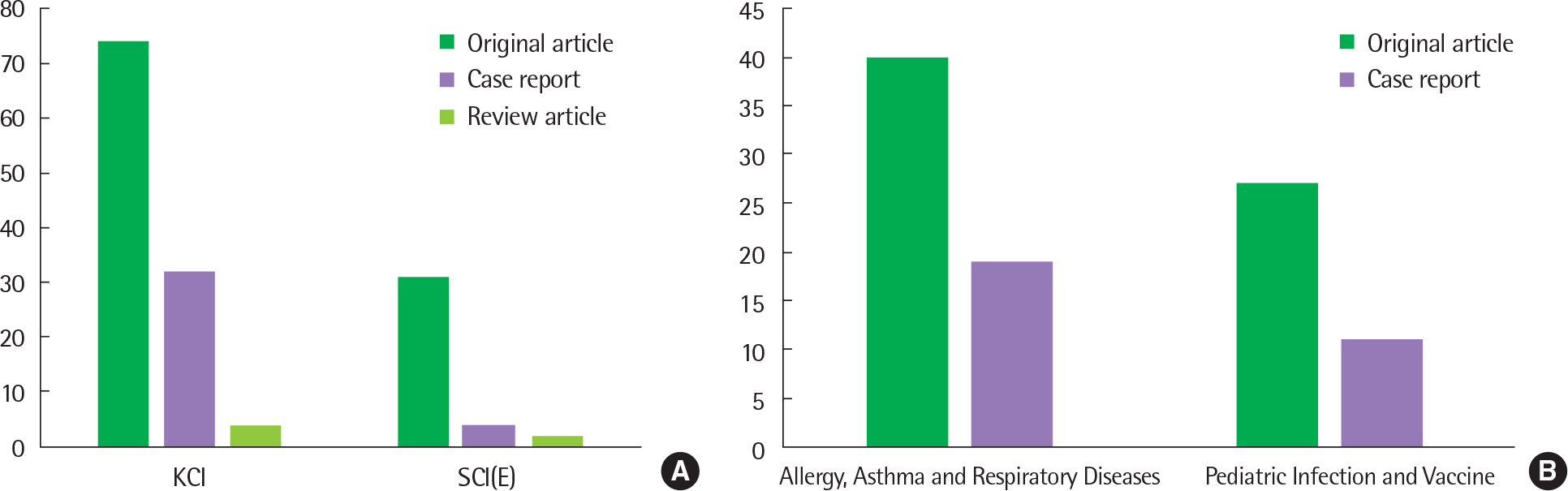
Fig. 3.
Number of pneumonia visits to medical institutions. Aadapted from National Health Insurance Corporation database.
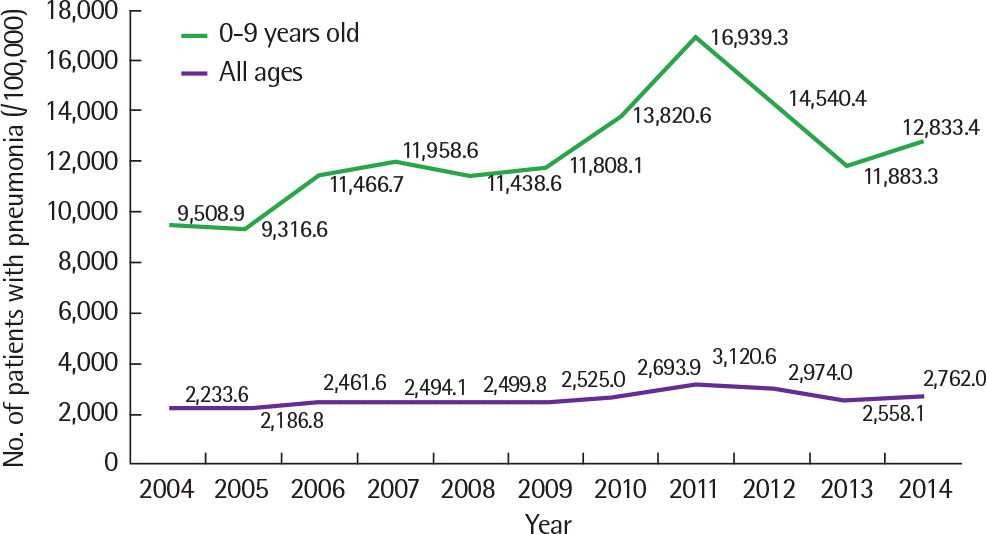
Fig. 4.
Number of death from pneumonia in Korean children. Aadapted from National Health Insurance Corporation database.
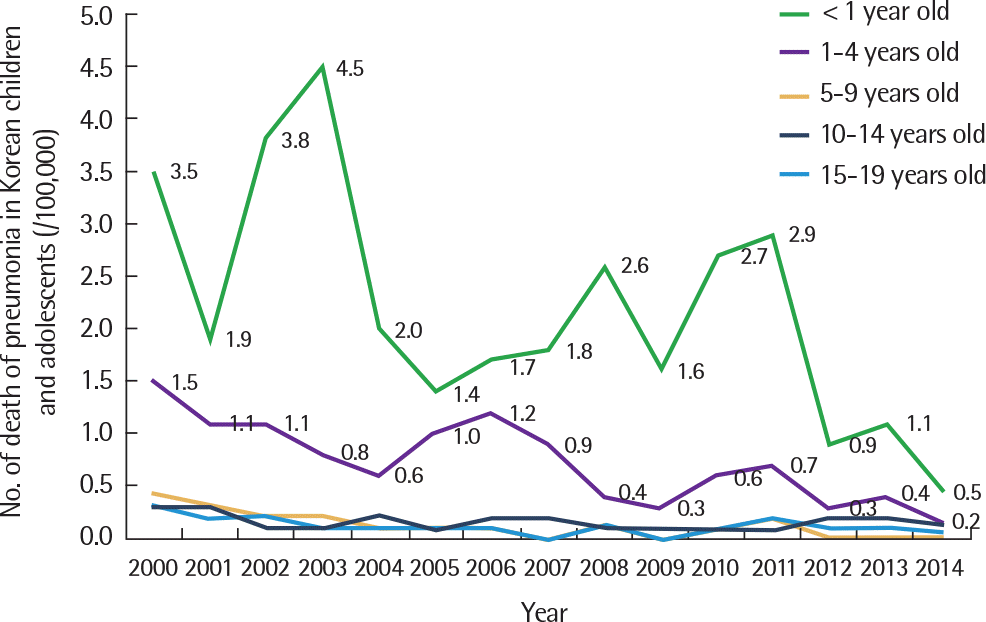
Fig. 5.
Changes of pneumonia mortality in Korean children before and after in-troduction of pneumococcal vaccine (from 1.2 to 0.5 per 100,000 in 1–4 years old age group; P=0.0175).
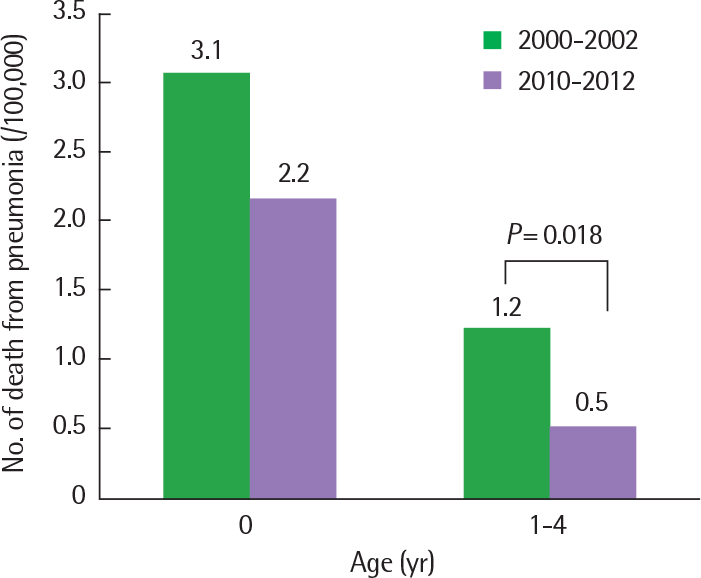
Table 1.
Empiric therapy for pediatric community-acquired pneumonia




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download