Abstract
The concept of personalized medicine for disease diagnosis, treatment, and management, considering individual variability, including susceptibility, clinical manifestations, and drug responsiveness, is a global emerging trend in medicine, which is also inevitable. However, clinical applications of personalized medicine in the real-world practice have been limited to certain cancers so far. Furthermore, this new concept to the diagnosis and treatment of adult asthma has not been applied to clinical use. Asthma is a multifactorial and heterogeneous disease. It seems to encompass a broad spectrum of clinical manifestations with different underlying pathophysiological mechanisms. Thus, it is not easy to categorize by their clinical features alone. Endotypical categorization that considering specific pathophysiological mechanisms will be more helpful in applying the concept of personalized medicine. The success of personalized medicine depends on patient selection for precise prescription of asthma medications. In the recent years, many investigators and physicians have devoted a lot of effort to the discovery of reliable biomarkers in asthmatic patients, which will be able to actualize the personalized medicine in near future. Despite such great efforts toward investigation of good biomarkers, few things have turned out to be practical in the clinic. Easily interpretable biomarkers of asthma are necessary to assess early detection, determination of treatment, prognosis prediction, and monitoring of exacerbation. Herein, we review recent studies regarding disease classifications and biomarkers of asthma.
Figures and Tables
Fig. 1
The schematic presentation of the underlying pathogenesis of allergic asthma. (A) The progress of allergic asthma demanded sensitization to an environmental allergen. Primary exposure to an allergen conducted activation of Th2 lymphocytes and ILC2. (B) Subsequently, re-exposure causes immediate reaction of the increasing production of Th2 cytokines, stimulation of IgE synthesis and accumulation of inflammatory cells in airway. PRR, pattern recognition receptor; IL, interleukin; ILC2, type 2 innate lymphoid cell; DC, dendritic cell.
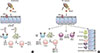
Table 2
Summary of biomarkers in adult asthma for clinical purposes

References
1. Lai CK, Beasley R, Crane J, Foliaki S, Shah J, Weiland S, et al. Global variation in the prevalence and severity of asthma symptoms: phase three of the International Study of Asthma and Allergies in Childhood (ISAAC). Thorax. 2009; 64:476–483.

2. Kim CY, Park HW, Ko SK, Chang SI, Moon HB, Kim YY, et al. The financial burden of asthma: a nationwide comprehensive survey conducted in the republic of Korea. Allergy Asthma Immunol Res. 2011; 3:34–38.

3. Anandan C, Nurmatov U, van Schayck OC, Sheikh A. Is the prevalence of asthma declining? Systematic review of epidemiological studies. Allergy. 2010; 65:152–167.

4. Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010; 181:315–323.

5. Sutherland ER, Goleva E, King TS, Lehman E, Stevens AD, Jackson LP, et al. Cluster analysis of obesity and asthma phenotypes. PLoS One. 2012; 7:e36631.

7. Lipworth BJ. Systemic adverse effects of inhaled corticosteroid therapy: a systematic review and meta-analysis. Arch Intern Med. 1999; 159:941–955.

9. Busse WW. Asthma diagnosis and treatment: filling in the information gaps. J Allergy Clin Immunol. 2011; 128:740–750.

10. Soukhanov AH. Encarta world English dictionary. New York: St Martin's Press;1999.
11. Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012; 18:716–725.

12. Lee HY, Kang JY, Yoon HK, Lee SY, Kwon SS, Kim YK, et al. Clinical characteristics of asthma combined with COPD feature. Yonsei Med J. 2014; 55:980–986.

13. Kim TB, Jang AS, Kwon HS, Park JS, Chang YS, Cho SH, et al. Identification of asthma clusters in two independent Korean adult asthma cohorts. Eur Respir J. 2013; 41:1308–1314.

14. Bourdin A, Molinari N, Vachier I, Varrin M, Marin G, Gamez AS, et al. Prognostic value of cluster analysis of severe asthma phenotypes. J Allergy Clin Immunol. 2014; 134:1043–1050.

15. Fingleton J, Travers J, Williams M, Charles T, Bowles D, Strik R, et al. Treatment responsiveness of phenotypes of symptomatic airways obstruction in adults. J Allergy Clin Immunol. 2015; 136:601–609.

16. Corren J. Asthma phenotypes and endotypes: an evolving paradigm for classification. Discov Med. 2013; 15:243–249.
17. Bartminski G, Crossley M, Turcanu V. Novel biomarkers for asthma stratification and personalized therapy. Expert Rev Mol Diagn. 2015; 15:415–430.

18. Anderson GP. Endotyping asthma: new insights into key pathogenic mechanisms in a complex, heterogeneous disease. Lancet. 2008; 372:1107–1119.

19. De Gruttola VG, Clax P, DeMets DL, Downing GJ, Ellenberg SS, Friedman L, et al. Considerations in the evaluation of surrogate endpoints in clinical trials. summary of a National Institutes of Health workshop. Control Clin Trials. 2001; 22:485–502.

20. Bousquet J, Chanez P, Lacoste JY, Barnéon G, Ghavanian N, Enander I, et al. Eosinophilic inflammation in asthma. N Engl J Med. 1990; 323:1033–1039.

21. Marko-Varga GA, Nilsson J, Laurell T. New directions of miniaturization within the biomarker research area. Electrophoresis. 2004; 25:3479–3491.

22. Deckers J, Branco Madeira F, Hammad H. Innate immune cells in asthma. Trends Immunol. 2013; 34:540–547.

23. Cayrol C, Girard JP. IL-33: an alarmin cytokine with crucial roles in innate immunity, inflammation and allergy. Curr Opin Immunol. 2014; 31:31–37.

24. Suzukawa M, Morita H, Nambu A, Arae K, Shimura E, Shibui A, et al. Epithelial cell-derived IL-25, but not Th17 cell-derived IL-17 or IL-17F, is crucial for murine asthma. J Immunol. 2012; 189:3641–3652.

25. Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+) Sca-1(+) lymphoid cells. Nature. 2010; 463:540–544.

26. Sibilano R, Frossi B, Pucillo CE. Mast cell activation: a complex interplay of positive and negative signaling pathways. Eur J Immunol. 2014; 44:2558–2566.

28. Jacobsen EA, Zellner KR, Colbert D, Lee NA, Lee JJ. Eosinophils regulate dendritic cells and Th2 pulmonary immune responses following allergen provocation. J Immunol. 2011; 187:6059–6068.

29. Schuijs MJ, Willart MA, Hammad H, Lambrecht BN. Cytokine targets in airway inflammation. Curr Opin Pharmacol. 2013; 13:351–361.

30. Woodruff PG, Modrek B, Choy DF, Jia G, Abbas AR, Ellwanger A, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009; 180:388–395.

31. Nakagome K, Matsushita S, Nagata M. Neutrophilic inflammation in severe asthma. Int Arch Allergy Immunol. 2012; 158:Suppl 1. 96–102.

32. Durrant DM, Metzger DW. Emerging roles of T helper subsets in the pathogenesis of asthma. Immunol Invest. 2010; 39:526–549.

33. Haldar P, Brightling CE, Hargadon B, Gupta S, Monteiro W, Sousa A, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009; 360:973–984.

34. Lim S, Jatakanon A, Meah S, Oates T, Chung KF, Barnes PJ. Relationship between exhaled nitric oxide and mucosal eosinophilic inflammation in mild to moderately severe asthma. Thorax. 2000; 55:184–188.

35. Crimi E, Spanevello A, Neri M, Ind PW, Rossi GA, Brusasco V. Dissociation between airway inflammation and airway hyperresponsiveness in allergic asthma. Am J Respir Crit Care Med. 1998; 157:4–9.

36. Loutsios C, Farahi N, Porter L, Lok LS, Peters AM, Condliffe AM, et al. Biomarkers of eosinophilic inflammation in asthma. Expert Rev Respir Med. 2014; 8:143–150.

37. Halonen M, Barbee RA, Lebowitz MD, Burrows B. An epidemiologic study of interrelationships of total serum immunoglobulin E, allergy skin-test reactivity, and eosinophilia. J Allergy Clin Immunol. 1982; 69:221–228.

38. Sherrill DL, Lebowitz MD, Halonen M, Barbee RA, Burrows B. Longitudinal evaluation of the association between pulmonary function and total serum IgE. Am J Respir Crit Care Med. 1995; 152:98–102.

39. Simpson A, Soderstrom L, Ahlstedt S, Murray CS, Woodcock A, Custovic A. IgE antibody quantification and the probability of wheeze in preschool children. J Allergy Clin Immunol. 2005; 116:744–749.

40. Wenzel S, Ford L, Pearlman D, Spector S, Sher L, Skobieranda F, et al. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med. 2013; 368:2455–2466.

41. Corren J, Lemanske RF, Hanania NA, Korenblat PE, Parsey MV, Arron JR, et al. Lebrikizumab treatment in adults with asthma. N Engl J Med. 2011; 365:1088–1098.

42. Szefler SJ, Wenzel S, Brown R, Erzurum SC, Fahy JV, Hamilton RG, et al. Asthma outcomes: biomarkers. J Allergy Clin Immunol. 2012; 129:3 Suppl. S9–23.

43. Baldacci S, Omenaas E, Oryszczyn MP. Allergy markers in respiratory epidemiology. Eur Respir J. 2001; 17:773–790.

44. Hastie AT, Moore WC, Li H, Rector BM, Ortega VE, Pascual RM, et al. Biomarker surrogates do not accurately predict sputum eosinophil and neutrophil percentages in asthmatic subjects. J Allergy Clin Immunol. 2013; 132:72–80.

45. Druilhe A, Létuvé S, Pretolani M. Glucocorticoid-induced apoptosis in human eosinophils: mechanisms of action. Apoptosis. 2003; 8:481–495.
46. Massanari M, Holgate ST, Busse WW, Jimenez P, Kianifard F, Zeldin R. Effect of omalizumab on peripheral blood eosinophilia in allergic asthma. Respir Med. 2010; 104:188–196.

47. Castro M, Mathur S, Hargreave F, Boulet LP, Xie F, Young J, et al. Reslizumab for poorly controlled, eosinophilic asthma: a randomized, placebo-controlled study. Am J Respir Crit Care Med. 2011; 184:1125–1132.

48. Leckie MJ, ten Brinke A, Khan J, Diamant Z, O'Connor BJ, Walls CM, et al. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet. 2000; 356:2144–2148.

49. Valent P, Gleich GJ, Reiter A, Roufosse F, Weller PF, Hellmann A, et al. Pathogenesis and classification of eosinophil disorders: a review of recent developments in the field. Expert Rev Hematol. 2012; 5:157–176.

51. Vijverberg SJ, Hilvering B, Raaijmakers JA, Lammers JW, Maitland-van der Zee AH, Koenderman L. Clinical utility of asthma biomarkers: from bench to bedside. Biologics. 2013; 7:199–210.
52. Kips JC, Fahy JV, Hargreave FE, Ind PW, in't Veen JC. Methods for sputum induction and analysis of induced sputum: a method for assessing airway inflammation in asthma. Eur Respir J Suppl. 1998; 26:9S–12S.
53. Simpson JL, Scott R, Boyle MJ, Gibson PG. Inflammatory subtypes in asthma: assessment and identification using induced sputum. Respirology. 2006; 11:54–61.

54. Green RH, Brightling CE, McKenna S, Hargadon B, Parker D, Bradding P, et al. Asthma exacerbations and sputum eosinophil counts: a randomised controlled trial. Lancet. 2002; 360:1715–1721.

55. Berry M, Morgan A, Shaw DE, Parker D, Green R, Brightling C, et al. Pathological features and inhaled corticosteroid response of eosinophilic and non-eosinophilic asthma. Thorax. 2007; 62:1043–1049.

56. Fleming L, Wilson N, Regamey N, Bush A. Use of sputum eosinophil counts to guide management in children with severe asthma. Thorax. 2012; 67:193–198.

57. Woodruff PG, Khashayar R, Lazarus SC, Janson S, Avila P, Boushey HA, et al. Relationship between airway inflammation, hyperresponsiveness, and obstruction in asthma. J Allergy Clin Immunol. 2001; 108:753–758.

58. Lex C, Ferreira F, Zacharasiewicz A, Nicholson AG, Haslam PL, Wilson NM, et al. Airway eosinophilia in children with severe asthma: predictive values of noninvasive tests. Am J Respir Crit Care Med. 2006; 174:1286–1291.
59. Morris SM Jr, Billiar TR. New insights into the regulation of inducible nitric oxide synthesis. Am J Physiol. 1994; 266(6 Pt 1):E829–E839.

60. Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011; 184:602–615.

61. Amelink M, de Groot JC, de Nijs SB, Lutter R, Zwinderman AH, Sterk PJ, et al. Severe adult-onset asthma: a distinct phenotype. J Allergy Clin Immunol. 2013; 132:336–341.

62. McNicholl DM, Stevenson M, McGarvey LP, Heaney LG. The utility of fractional exhaled nitric oxide suppression in the identification of nonadherence in difficult asthma. Am J Respir Crit Care Med. 2012; 186:1102–1108.

63. Smith AD, Cowan JO, Brassett KP, Herbison GP, Taylor DR. Use of exhaled nitric oxide measurements to guide treatment in chronic asthma. N Engl J Med. 2005; 352:2163–2173.

64. Petsky HL, Cates CJ, Lasserson TJ, Li AM, Turner C, Kynaston JA, et al. A systematic review and meta-analysis: tailoring asthma treatment on eosinophilic markers (exhaled nitric oxide or sputum eosinophils). Thorax. 2012; 67:199–208.

65. Calhoun WJ, Ameredes BT, King TS, Icitovic N, Bleecker ER, Castro M, et al. Comparison of physician-, biomarker-, and symptom-based strategies for adjustment of inhaled corticosteroid therapy in adults with asthma: the BASALT randomized controlled trial. JAMA. 2012; 308:987–997.

66. Deykin A. Targeting biologic markers in asthma--is exhaled nitric oxide the bull's-eye? N Engl J Med. 2005; 352:2233–2235.

67. Gillan L, Matei D, Fishman DA, Gerbin CS, Karlan BY, Chang DD. Periostin secreted by epithelial ovarian carcinoma is a ligand for alpha(V)beta(3) and alpha(V)beta(5) integrins and promotes cell motility. Cancer Res. 2002; 62:5358–5364.
68. Sidhu SS, Yuan S, Innes AL, Kerr S, Woodruff PG, Hou L, et al. Roles of epithelial cell-derived periostin in TGF-beta activation, collagen production, and collagen gel elasticity in asthma. Proc Natl Acad Sci U S A. 2010; 107:14170–14175.

69. Woodruff PG, Boushey HA, Dolganov GM, Barker CS, Yang YH, Donnelly S, et al. Genome-wide profiling identifies epithelial cell genes associated with asthma and with treatment response to corticosteroids. Proc Natl Acad Sci U S A. 2007; 104:15858–15863.

70. Hanania NA, Wenzel S, Rosén K, Hsieh HJ, Mosesova S, Choy DF, et al. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med. 2013; 187:804–811.

71. Cai C, Yang J, Hu S, Zhou M, Guo W. Relationship between urinary cysteinyl leukotriene E4 levels and clinical response to antileukotriene treatment in patients with asthma. Lung. 2007; 185:105–112.

72. Chupp GL, Lee CG, Jarjour N, Shim YM, Holm CT, He S, et al. A chitinase-like protein in the lung and circulation of patients with severe asthma. N Engl J Med. 2007; 357:2016–2027.

73. Konradsen JR, James A, Nordlund B, Reinius LE, Söderhäll C, Melön E, et al. The chitinase-like protein YKL-40: a possible biomarker of inflammation and airway remodeling in severe pediatric asthma. J Allergy Clin Immunol. 2013; 132:328–335.e5.
74. Specjalski K, Chełmińska M, Jassem E. YKL-40 protein correlates with the phenotype of asthma. Lung. 2015; 193:189–194.

75. Ober C, Tan Z, Sun Y, Possick JD, Pan L, Nicolae R, et al. Effect of variation in CHI3L1 on serum YKL-40 level, risk of asthma, and lung function. N Engl J Med. 2008; 358:1682–1691.

76. Fens N, Roldaan AC, van der Schee MP, Boksem RJ, Zwinderman AH, Bel EH, et al. External validation of exhaled breath profiling using an electronic nose in the discrimination of asthma with fixed airways obstruction and chronic obstructive pulmonary disease. Clin Exp Allergy. 2011; 41:1371–1378.

77. Dragonieri S, Schot R, Mertens BJ, Le Cessie S, Gauw SA, Spanevello A, et al. An electronic nose in the discrimination of patients with asthma and controls. J Allergy Clin Immunol. 2007; 120:856–862.

78. van der Schee MP, Palmay R, Cowan JO, Taylor DR. Predicting steroid responsiveness in patients with asthma using exhaled breath profiling. Clin Exp Allergy. 2013; 43:1217–1225.

79. Horvath I, Hunt J, Barnes PJ, Alving K, Antczak A, Baraldi E, et al. Exhaled breath condensate: methodological recommendations and unresolved questions. Eur Respir J. 2005; 26:523–548.

80. Tufvesson E, Bjermer L. Methodological improvements for measuring eicosanoids and cytokines in exhaled breath condensate. Respir Med. 2006; 100:34–38.

81. Montuschi P, Barnes PJ. Exhaled leukotrienes and prostaglandins in asthma. J Allergy Clin Immunol. 2002; 109:615–620.

82. Fireman E, Shtark M, Priel IE, Shiner R, Mor R, Kivity S, et al. Hydrogen peroxide in exhaled breath condensate (EBC) vs eosinophil count in induced sputum (IS) in parenchymal vs airways lung diseases. Inflammation. 2007; 30:44–51.

83. Koh GC, Shek LP, Goh DY, Van Bever H, Koh DS. Eosinophil cationic protein: is it useful in asthma? A systematic review. Respir Med. 2007; 101:696–705.

84. Bartoli ML, Bacci E, Carnevali S, Cianchetti S, Dente FL, Di Franco A, et al. Clinical assessment of asthma severity partially corresponds to sputum eosinophilic airway inflammation. Respir Med. 2004; 98:184–193.

85. Prehn A, Seger RA, Torresani T, Molinari L, Sennhauser FH. Evaluation of a clinical algorithm involving serum eosinophil cationic protein for guiding the anti-inflammatory treatment of bronchial asthma in childhood. Pediatr Allergy Immunol. 2000; 11:87–94.

86. Kumar SD, Emery MJ, Atkins ND, Danta I, Wanner A. Airway mucosal blood flow in bronchial asthma. Am J Respir Crit Care Med. 1998; 158:153–156.

87. García G, Bergna M, Uribe E, Yañez A, Soriano JB. Increased exhaled breath temperature in subjects with uncontrolled asthma. Int J Tuberc Lung Dis. 2013; 17:969–972.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download