This article has been corrected. See "Erratum: Acknowledgments Addition. Validation of “quality-of-life questionnaire in Korean children with allergic rhinitis” in middle school students" in Volume 4 on page 463.
Abstract
Purpose
The quality of life questionnaire in Korean children with allergic rhinitis (QoL-KCAR) was developed to assess the quality of life in Korean children aged 6–12 years old with allergic rhinitis. The aim of this study was to validate the QoL-KCAR in Korean middle school students with allergic rhinitis.
Methods
A survey with questionnaire and skin prick test was performed on 277 middle school students. The students were classified into 3 groups: allergic-rhinitis (AR), non-allergic rhinitis (non-AR), and controls. AR was defined who had nasal symptoms within 12 months and positive response to skin test. Non-AR group was composed of students with nasal symptoms but had negative response to skin test. The rest who had no symptoms of rhinitis and negative response to skin test were classed as control group. QoL-KCAR has 10 questions with 5-point scales for response options.
Results
There were significant differences in the answer scores among the 3 groups (P<0.05). Total answer score is 20.9±10.2, 17.3±8.8, 4.4±5.6 in the AR, non-AR group, and control groups, respectively. It showed significant differences in all items between the AR and control groups, and 4 questions between the AR and non-AR groups. There were significant differences in total score and each score of 8 questions between before and after education in AR group.
Figures and Tables
Table 1
Mean scores of QoL-KCAR

Table 2
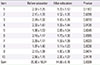
Least squares means in all groups

| Score before education | Least squares mean after education | |||
|---|---|---|---|---|
| AR | Non-AR | Control | P-value | |
| 15 | 9.95 | 9.47 | 5.83 | < 0.0001 |
| 25 | 17.76 | 16.10 | 9.90 | < 0.0001 |
| 40 | 29.47 | 26.03 | 16.02 | < 0.0001 |
Table 3
Mean score of QoL-KCAR before and after education in AR group
References
1. Hong SJ, Ahn KM, Lee SY, Kim KE. The prevalences of asthma and allergic diseases in Korean children. Korean J Pediatr. 2008; 51:343–350.

2. Ahn K, Kim J, Kwon HJ, Chae Y, Hahm MI, Lee KJ, et al. The prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in Korean children: nationwide cross-sectional survey using complex sampling design. J Korean Med Assoc. 2011; 54:769–778.

3. Juniper EF, Guyatt GH. Development and testing of a new measure of health status for clinical trials in rhinoconjunctivitis. Clin Exp Allergy. 1991; 21:77–83.

4. Juniper EF, Thompson AK, Ferrie PJ, Roberts JN. Validation of the standardized version of the Rhinoconjunctivitis Quality of Life Questionnaire. J Allergy Clin Immunol. 1999; 104(2 Pt 1):364–369.

5. Juniper EF, Thompson AK, Ferrie PJ, Roberts JN. Development and validation of the mini Rhinoconjunctivitis Quality of Life Questionnaire. Clin Exp Allergy. 2000; 30:132–140.

6. Organization for Economic Co-operation and Development. Quality time for students: learning in and out of school. Paris: OECD Publishing;2011. p. 22–36.
7. Kim JH, Ahn YM, Kim HJ, Lim DH, Son BK, Kang HS, et al. Development of a questionnaire for the assessment of quality of life in korean children with allergic rhinitis. Allergy Asthma Immunol Res. 2014; 6:541–547.

8. Position paper: Allergen standardization and skin tests. The European Academy of Allergology and Clinical Immunology. Allergy. 1993; 48:14 Suppl. 48–82.
10. Skoner DP. Allergic rhinitis: definition, epidemiology, pathophysiology, detection, and diagnosis. J Allergy Clin Immunol. 2001; 108:1 Suppl. S2–S8.

11. Park KH, Cho JS, Lee KH, Shin SY, Moon JH, Cha CI. Rhinoconjunctivitis quality of life questionnaire (RQLQ) as an evaluator of perennial allergic rhinitis patients: the first report. Korean J Otolaryngol-Head Neck Surg. 2002; 45:254–262.
12. Jin JY, Yang HJ, Jeon YH, Kim KW, Kim WK, Park YM, et al. Development and validation of the questionnaire for quality-of-life specific to allergic rhinitis in Korean children (QQOL-ARK): A Multicenter Study. Korean J Asthma Allergy Clin Immunol. 2009; 29:242–248.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download