Abstract
Purpose
It is recommended to use 200 (2 puffs) or 400 (4 puffs) µg of salbutamol in the bronchodilator response (BDR) test. We aimed to compare the difference between these 2 doses with regard to small airway dysfunction.
Methods
One hundred sixteen subjects who visited the hospital for diagnosis or follow-up of asthma were consecutively enrolled between June 1 and November 31, 2013. The subjects were randomly assigned to the BDR test at the 2 doses (200 or 400 µg of salbutamol), with physicians blinded to the group each subject was assigned to and undertook the BDR test using the spirometry and impulse oscillometry system (IOS).
Results
A total of 116 subjects participated in this study; the mean age was 7.8±3.6 years. The number of participants who were assigned to 2 and 4 puffs groups was 59 and 57, respectively. The mean age was older in the 4 puffs group than in the 2 puffs group (P=0.008). There were no significant difference in spirometric and oscillometric parameters between the 2 and 4 puffs groups. However, in subgroup analysis of asthmatic patients on maintenance therapy (n=21), there was a significant difference in relative changes in Rrs5 between the 2 and 4 puffs groups (16.4%±9.6% vs. 28.7%±8.8%, P=0.035). The forced expiratory volume of 1 second showed a significant correlation with resistance in the 2 puffs group and with reactance in the 4 puffs group.
Conclusion
There was a significant relationship between the amounts of bronchodilators administered and the small airway dysfunction in children with asthma on maintenance therapy. Further research is warranted to delineate changes in spirometric and IOS measures in accordance with the different amounts of bronchodilators administered.
Figures and Tables
 | Fig. 1Changes in Rrs5 (A) and Xrs5 (B) of impulse oscillometry system according to changes in forced expiratory volume of 1 second (FEV1) of spirometry (r=0.216, P=0.021 vs. r=0.250, P=0.007). |
 | Fig. 2The dotted line on the x-axis represents a 12% change in forced expiratory volume of 1 second (FEV1) of spirometry and the one on the y-axis a 34% change in Rrs5 of impulse oscillometry system, thus showing where subjects are classified according to the 2 results. |
Table 1
Clinical characteristics of 2 puff and 4 puff dose with salbutamol for bronchodilator response
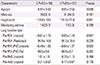
Table 2
Correlation of percent change of FEV1 and IOS parameter

Table 3
Difference of spiometric and oscillometric lung function test between the 2 puff and 4 puff with salbutamol

References
1. Tse SM, Gold DR, Sordillo JE, Hoffman EB, Gillman MW, Rifas-Shiman SL, et al. Diagnostic accuracy of the bronchodilator response in children. J Allergy Clin Immunol. 2013; 132:554–559.e5.

2. Tan WC, Vollmer WM, Lamprecht B, Mannino DM, Jithoo A, Nizankowska-Mogilnicka E, et al. Worldwide patterns of bronchodilator responsiveness: results from the Burden of Obstructive Lung Disease study. Thorax. 2012; 67:718–726.

3. Shin YH, Jang SJ, Yoon JW, Jee HM, Choi SH, Yum HY, et al. Oscillometric and spirometric bronchodilator response in preschool children with and without asthma. Can Respir J. 2012; 19:273–277.

4. The Global Initiative for Asthma. Global strategy for asthma management and prevention (2015 update) [Internet]. The Global Initiative for Asthma;cited 2016 Jan 17. Available from: http://www.ginasthma.org/documents/5/documents_variants/59.
5. Seo BS, Lee JM, Cho E, Baek JH, Lee GS, Shin YH, et al. Relationship between exhaled nitric oxide and small-airway dysfunction in children with asthma using spirometry and the impulse oscillometry system. Allergy Asthma Respir Dis. 2015; 3:267–271.

6. del Giudice MM, Brunese FP, Piacentini GL, Pedulla M, Capristo C, Decimo F, et al. Fractional exhaled nitric oxide (FENO), lung function and airway hyperresponsiveness in naïve atopic asthmatic children. J Asthma. 2004; 41:759–765.

7. Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005; 26:319–338.

8. Oostveen E, MacLeod D, Lorino H, Farre R, Hantos Z, Desager K, et al. The forced oscillation technique in clinical practice: methodology, recommendations and future developments. Eur Respir J. 2003; 22:1026–1041.

9. Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012; 40:1324–1343.

10. Frei J, Jutla J, Kramer G, Hatzakis GE, Ducharme FM, Davis GM. Impulse oscillometry: reference values in children 100 to 150 cm in height and 3 to 10 years of age. Chest. 2005; 128:1266–1273.
11. Calogero C, Simpson SJ, Lombardi E, Parri N, Cuomo B, Palumbo M, et al. Respiratory impedance and bronchodilator responsiveness in healthy children aged 2-13 years. Pediatr Pulmonol. 2013; 48:707–715.

12. Oostveen E, Boda K, van der Grinten CP, James AL, Young S, Nieland H, et al. Respiratory impedance in healthy subjects: baseline values and bronchodilator response. Eur Respir J. 2013; 42:1513–1523.

13. López-Campos JL, Soriano JB, Calle M. Encuesta de Espirometría en España (3E) Project. A comprehensive, national survey of spirometry in Spain: current bottlenecks and future directions in primary and secondary care. Chest. 2013; 144:601–609.

14. Nelson HS, Spector SL, Whitsett TL, George RB, Dwek JH. The bronchodilator response to inhalation of increasing doses of aerosolized albuterol. J Allergy Clin Immunol. 1983; 72:371–375.

15. Denjean A, Guimaraes H, Migdal M, Miramand JL, Dehan M, Gaultier C. Dose-related bronchodilator response to aerosolized salbutamol (albuterol) in ventilator-dependent premature infants. J Pediatr. 1992; 120:974–979.

16. Rodriguez-Carballeira M, Heredia JL, Rue M, Quintana S, Gomez L. The bronchodilator test with increasing doses of terbutaline in chronic obstructive pulmonary disease patients. Pulm Pharmacol Ther. 2001; 14:61–65.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download