Abstract
Purpose
We aimed to translate the Test for Respiratory and Asthma Control in Kids (TRACK) instrument into Korean, with subsequent linguistic validation.
Methods
The multistep process of forward translation, reconciliation, back-translation, cognitive debriefing, and proofreading of the Korean version of the TRACK was completed.
Results
Two bilingual medical personnel independently translated the original English version of the TRACK into Korean one. After moderating the translation into a single reconciled one, 4 other bilingual persons were invited to translate the Korean draft back into an English one. Discrepancies between the original English version and the back-translated one were reviewed, and the need to modify the reconciled Korean draft was discussed. Twenty caregivers of asthmatic children took part in interviews that examine the appropriateness of the Korean version of the TRACK. The feedback from caregivers were then reviewed by a panel of pediatric allergists and reflected in the final Korean version. The document was finally proofread to check the spelling, grammar, layout and formatting.
Figures and Tables
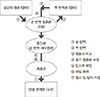 | Fig. 1Translation and linguistic validation process of the questionnaire. After ① translation of the original version of the questionnaire into the target language, ② back translation, ③ comparison with the original version, and ④ modification of the Korean draft are followed. Thereafter, ⑤ cognitive debriefing is conducted with interviewee who would answer the questionnaire and ⑥ the result is reviewed by a panel of pediatric allergists. Finally, ⑦ checking for spelling, grammar and formatting was done to complete the harmonized translation. |
Table 1
Reconciliation after two forward translations of the Test for Respiratory and Asthma Control in Kids by two independent bilingual translators

References
1. National Asthma Education and Prevention Program. Expert Panel report 2: guidelines for the diagnosis and management of asthma 1997. Bethesda (MD): U.S. Department of Health & Human Services, NIH logoNational Institutes of Health, National Heart, Lung, and Blood Institute;1997.
2. The Global Initiative for Asthma. 2002 Original: Workshop Report, Global Strategy for Asthma Management and Prevention [Internet]. The Global Initiative for Asthma;cited 2015 Jul 15. Available from: http://www.ginasthma.org/documents/5/documents_variants/21.
3. Reddel HK, Levy ML. Global Initiative for Asthma Scientific Committee and Dissemination and Implementation Committee. The GINA asthma strategy report: what's new for primary care? NPJ Prim Care Respir Med. 2015; 25:15050.

4. The Global Initiative for Asthma. 2006 Revision: GINA report, global strategy for asthma management and prevention [Internet]. The Global Initiative for Asthma;cited 2015 Aug 31. Available from: http://www.ginasthma.org/documents/5/documents_variants/31.
5. Covar RA, Strunk R, Zeiger RS, Wilson LA, Liu AH, Weiss S, et al. Predictors of remitting, periodic, and persistent childhood asthma. J Allergy Clin Immunol. 2010; 125:359–366.e3.

6. Bisgaard H, Bonnelykke K. Long-term studies of the natural history of asthma in childhood. J Allergy Clin Immunol. 2010; 126:187–197.

7. Guilbert TW, Mauger DT, Allen DB, Zeiger RS, Lemanske RF Jr, Szefler SJ, et al. Growth of preschool children at high risk for asthma 2 years after discontinuation of fluticasone. J Allergy Clin Immunol. 2011; 128:956–963.e1-7.

8. Kelly HW, Sternberg AL, Lescher R, Fuhlbrigge AL, Williams P, Zeiger RS, et al. Effect of inhaled glucocorticoids in childhood on adult height. N Engl J Med. 2012; 367:904–912.

9. Nathan RA, Sorkness CA, Kosinski M, Schatz M, Li JT, Marcus P, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004; 113:59–65.

10. Murphy KR, Zeiger RS, Kosinski M, Chipps B, Mellon M, Schatz M, et al. Test for respiratory and asthma control in kids (TRACK): a caregiver-completed questionnaire for preschool-aged children. J Allergy Clin Immunol. 2009; 123:833–839.e9.

11. Skinner EA, Diette GB, Algatt-Bergstrom PJ, Nguyen TT, Clark RD, Markson LE, et al. The Asthma Therapy Assessment Questionnaire (ATAQ) for children and adolescents. Dis Manag. 2004; 7:305–313.

12. Juniper EF, O'Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999; 14:902–907.

13. Liu AH, Zeiger R, Sorkness C, Mahr T, Ostrom N, Burgess S, et al. Development and cross-sectional validation of the Childhood Asthma Control Test. J Allergy Clin Immunol. 2007; 119:817–825.

14. Chipps B, Zeiger RS, Murphy K, Mellon M, Schatz M, Kosinski M, et al. Longitudinal validation of the Test for Respiratory and Asthma Control in Kids in pediatric practices. Pediatrics. 2011; 127:e737–e747.

15. Chipps BE, Mellon MM, Murphy KR, Zeiger RS. Test for respiratory and asthma control in kids (TRACK): a validated control tool for preschool-aged children. J Allergy Clin Immunol. 2014; 133:1776.

16. Deschildre A, Pin I, El Abd K, Belmin-Larrar S, El Mourad S, Thumerelle C, et al. Asthma control assessment in a pediatric population: comparison between GINA/NAEPP guidelines, Childhood Asthma Control Test (C-ACT), and physician's rating. Allergy. 2014; 69:784–790.

17. Cloutier MM, Schatz M, Castro M, Clark N, Kelly HW, Mangione-Smith R, et al. Asthma outcomes: composite scores of asthma control. J Allergy Clin Immunol. 2012; 129:3 Suppl. S24–S33.

18. Zeiger RS, Mellon M, Chipps B, Murphy KR, Schatz M, Kosinski M, et al. Test for Respiratory and Asthma Control in Kids (TRACK): clinically meaningful changes in score. J Allergy Clin Immunol. 2011; 128:983–988.

19. Linguistic validation of the PedsQLTM - a quality of life questionnaire [Internet]. Lyon: Mapi Research Trust;c1998-2016. cited 2015 Aug 31. Available from: http://www.pedsql.org/PedsQL-Linguistic-Validation-Guidelines.doc.
20. BITDruginfo [Internet]. Seoul: BIT computer;cited 2015 Jul 15. Available from: https://www.druginfo.co.kr/.
21. The Global Initiative for Asthma. Global strategy for asthma management and prevention (2015 update) [Internet]. The Global Initiative for Asthma;2015 update. cited 2015 Jul 15. Available from: http://www.ginasthma.org/documents/5/documents_variants/59.
22. Rodríguez-Martínez CE, Nino G, Castro-Rodriguez JA. Validation of the Spanish version of the Test for Respiratory and Asthma Control in Kids (TRACK) in a population of Hispanic preschoolers. J Allergy Clin Immunol Pract. 2014; 2:326–331.e3.

23. Malmkjaer K. Linguistics and the language of translation. Chippenham: Atiny Rowe Ltd.;2005.
24. Linguistic Validation [Internet]. East Hartford (CT): Corporate Translations;cited 2015 Jun 14. Available from: http://www.corptransinc.com/Services/Linguistic-Validation.aspx.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download