Abstract
Hypersensitivity reactions to antituberculosis medicine are obstacles to the treatment of tuberculosis. However, rapid drug desensitization can secure successful treatment with essential antituberculosis medicines in pediatric patients. A 17-year-old boy with active pulmonary tuberculosis complained of generalized erythematous rashes, pruritus on the 11th day of tuberculosis treatment. He was diagnosed with hypersensitivity reactions to isoniazid and rifampin by the oral provocation test. After desensitization, the patient continued to take antituberculosis treatment with isoniazid, rifampin, pyrazinamide, and ethambutol. We report here a case of successful desensitization in an adolescent with hypersensitivity to isoniazid and rifampin.
Figures and Tables
Table 1
The result of skin test of rifampin and isoniazid
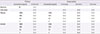
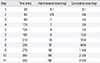
Table 2
Oral desensitization protocol for rifampin

References
2. Korea Centers for Disease Control & Prevention. Annual report on the tuberculosis cases notified in Korea 2013. Cheongwon: Korean Centers for Disease Control & Prevention;2014.
3. Kuyucu S, Mori F, Atanaskovic-Markovic M, Caubet JC, Terreehorst I, Gomes E, et al. Hypersensitivity reactions to non-betalactam antibiotics in children: an extensive review. Pediatr Allergy Immunol. 2014; 25:534–543.

4. Lee AR, Kim SJ, Kim J, Park JH, Lee JK, Kim JY, et al. Successful desensitization for antitubercular drugs. Allergy Asthma Respir Dis. 2013; 1:395–399.

5. Panova LV, Ovsiankina ES. Incidence of adverse reactions to chemotherapy and their types in adolescents with tuberculosis. Probl Tuberk. 2003; (1):28–30.
6. Holland CL, Malasky C, Ogunkoya A, Bielory L. Rapid oral desensitization to isoniazid and rifampin. Chest. 1990; 98:1518–1519.

7. Abadoglu O, Epozturk K, Atayik E. Rapid oral desensitisation to prophylactic isoniazid. Allergol Immunopathol (Madr). 2011; 39:311–312.

8. Kim JH, Kim HB, Kim BS, Hong SJ. Rapid oral desensitization to isoniazid, rifampin, and ethambutol. Allergy. 2003; 58:540–541.

9. de Groot H, Mulder WM. Clinical practice: drug desensitization in children. Eur J Pediatr. 2010; 169:1305–1309.
10. Forget EJ, Menzies D. Adverse reactions to first-line antituberculosis drugs. Expert Opin Drug Saf. 2006; 5:231–249.

11. Scherer K, Brockow K, Aberer W, Gooi JH, Demoly P, Romano A, et al. Desensitization in delayed drug hypersensitivity reactions: an EAACI position paper of the Drug Allergy Interest Group. Allergy. 2013; 68:844–852.

13. Kobashi Y, Okimoto N, Matsushima T, Abe T, Nishimura K, Shishido S, et al. Desensitization therapy for allergic reactions of antituberculous drugs: evaluation of desensitization therapy according to the guideline of the Japanese Society for Tuberculosis. Kekkaku. 2000; 75:699–704.
14. Hildebrand KJ, Atkinson A, Kitai I. Rifampin hypersensitivity in a 2-year-old child with successful rapid oral desensitization. Pediatr Infect Dis J. 2014; 33:787.

15. Logsdon S, Ramirez-Avila L, Castells M, Dioun A. Successful rifampin desensitization in a pediatric patient with latent tuberculosis. Pediatr Allergy Immunol. 2014; 25:404–405.

16. Portnoy J, Bagstad K, Kanarek H, Pacheco F, Hall B, Barnes C. Premedication reduces the incidence of systemic reactions during inhalant rush immunotherapy with mixtures of allergenic extracts. Ann Allergy. 1994; 73:409–418.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download