Abstract
Allergic airway diseases are characterized by T-helper type 2 (Th2)-skewed eosinophilic inflammation, mucus hypersecretion, and airway hyperresponsiveness. The excessive activation of Th2 cells due to insufficient suppression of regulatory T cells (Tregs) is thought to play a major role in the initiation and development of allergic airway disease. Several studies have shown that stem cells provide a significant reduction in allergic airway inflammation and improve lung function in animal models. The immunomodulatory effects of stem cells in allergic airway disease may be mediated by the upregulation of Tregs and increases in several soluble factors, such as prostaglandin E2, transforming growth factor-β, interleukin-10, and indoleamine 2, 3-dioxygenase. This review ex-amines the current understanding of the immunomodulatory properties of stem cells and its therapeutic implication in allergic airway disease. Furthermore, we will discuss mechanisms by which stem cells inhibit allergic airway inflammation via immunomodulation from a Th2- to a Th1-biased response.
Go to : 
References
1. Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008; 8:726–36.

2. Tuan RS, Boland G, Tuli R. Adult mesenchymal stem cells and cell-based tissue engineering. Arthritis Res Ther. 2003; 5:32–45.
3. Law S, Chaudhuri S. Mesenchymal stem cell and regenerative medicine: regeneration versus immunomodulatory challenges. Am J Stem Cells. 2013; 2:22–38.
4. Delorme B, Charbord P. Culture and characterization of human bone marrow mesenchymal stem cells. Methods Mol Med. 2007; 140:67–81.

5. Liu TM, Martina M, Hutmacher DW, Hui JH, Lee EH, Lim B. Identification of common pathways mediating differentiation of bone marrow- and adipose tissue-derived human mesenchymal stem cells into three mesenchymal lineages. Stem Cells. 2007; 25:750–60.

6. Titorencu I, Jinga V, Constantinescu E, Gafencu A, Ciohodaru C, Mano-lescu I, et al. Proliferation, differentiation and characterization of osteoblasts from human BM mesenchymal cells. Cytotherapy. 2007; 9:682–96.

7. Bhagavati S, Xu W. Isolation and enrichment of skeletal muscle progenitor cells from mouse bone marrow. Biochem Biophys Res Commun. 2004; 318:119–24.

8. Shi M, Liu ZW, Wang FS. Immunomodulatory properties and therapeutic application of mesenchymal stem cells. Clin Exp Immunol. 2011; 164:1–8.

9. Ma S, Xie N, Li W, Yuan B, Shi Y, Wang Y. Immunobiology of mesenchymal stem cells. Cell Death Differ. 2014; 21:216–25.

10. Cho KS, Park HK, Park HY, Jung JS, Jeon SG, Kim YK, et al. IFATS collection: immunomodulatory effects of adipose tissue-derived stem cells in an allergic rhinitis mouse model. Stem Cells. 2009; 27:259–65.

11. Sun YQ, Deng MX, He J, Zeng QX, Wen W, Wong DS, et al. Human pluripotent stem cell-derived mesenchymal stem cells prevent allergic airway inflammation in mice. Stem Cells. 2012; 30:2692–9.

12. Fu QL, Chow YY, Sun SJ, Zeng QX, Li HB, Shi JB, et al. Mesenchymal stem cells derived from human induced pluripotent stem cells modulate T-cell phenotypes in allergic rhinitis. Allergy. 2012; 67:1215–22.

13. Park HK, Cho KS, Park HY, Shin DH, Kim YK, Jung JS, et al. Adipose-derived stromal cells inhibit allergic airway inflammation in mice. Stem Cells Dev. 2010; 19:1811–8.

14. Ge X, Bai C, Yang J, Lou G, Li Q, Chen R. Intratracheal transplantation of bone marrowderived mesenchymal stem cells reduced airway inflammation and upregulated CD4+ CD25+ regulatory T cells in asthmatic mouse. Cell Biol Int. 2013; 37:675–86.
15. Ge X, Bai C, Yang J, Lou G, Li Q, Chen R. Effect of mesenchymal stem cells on inhibiting airway remodeling and airway inflammation in chronic asthma. J Cell Biochem. 2013; 114:1595–605.

16. Boulay ME, Boulet LP. The relationships between atopy, rhinitis and asthma: pathophysiological considerations. Curr Opin Allergy Clin Immunol. 2003; 3:51–5.

17. Togias A. Rhinitis and asthma: evidence for respiratory system integration. J Allergy Clin Immunol. 2003; 111:1171–83.

18. Wilson MS, Taylor MD, Balic A, Finney CA, Lamb JR, Maizels RM. Suppression of allergic airway inflammation by helminth-induced regulatory T cells. J Exp Med. 2005; 202:1199–212.

19. Shi HZ, Qin XJ. CD4CD25 regulatory T lymphocytes in allergy and asthma. Allergy. 2005; 60:986–95.

20. Jaffar Z, Sivakuru T, Roberts K. CD4+CD25+ T cells regulate airway eosinophilic inflammation by modulating the Th2 cell phenotype. J Immunol. 2004; 172:3842–9.

21. Puissant B, Barreau C, Bourin P, Clavel C, Corre J, Bousquet C, et al. Immunomodulatory effect of human adipose tissue-derived adult stem cells: comparison with bone marrow mesenchymal stem cells. Br J Haematol. 2005; 129:118–29.

22. Yanez R, Lamana ML, García-Castro J, Colmenero I, Ramirez M, Bueren JA. Adipose tissue-derived mesenchymal stem cells have in vivo immunosuppressive properties applicable for the control of the graft-versus-host disease. Stem Cells. 2006; 24:2582–91.

23. Glennie S, Soeiro I, Dyson PJ, Lam EW, Dazzi F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005; 105:2821–7.

24. Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006; 107:367–72.

25. Ramasamy R, Fazekasova H, Lam EW, Soeiro I, Lombardi G, Dazzi F. Mesenchymal stem cells inhibit dendritic cell differentiation and function by preventing entry into the cell cycle. Transplantation. 2007; 83:71–6.

26. English K, Barry FP, Mahon BP. Murine mesenchymal stem cells suppress dendritic cell migration, maturation and antigen presentation. Immunol Lett. 2008; 115:50–8.

27. Cohn L, Elias JA, Chupp GL. Asthma: mechanisms of disease persistence and progression. Annu Rev Immunol. 2004; 22:789–815.

28. Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005; 105:1815–22.

29. Beyth S, Borovsky Z, Mevorach D, Liebergall M, Gazit Z, Aslan H, et al. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood. 2005; 105:2214–9.

30. Sato K, Ozaki K, Oh I, Meguro A, Hatanaka K, Nagai T, et al. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood. 2007; 109:228–34.

31. Cui L, Yin S, Liu W, Li N, Zhang W, Cao Y. Expanded adipose-derived stem cells suppress mixed lymphocyte reaction by secretion of prostaglandin E2. Tissue Eng. 2007; 13:1185–95.

32. Cho KS, Roh HJ. Immunomodulatory effects of adipose-derived stem cells in airway allergic diseases. Curr Stem Cell Res Ther. 2010; 5:111–5.

33. Sueblinvong V, Weiss DJ. Stem cells and cell therapy approaches in lung biology and diseases. Transl Res. 2010; 156:188–205.

34. Cho KS, Park MK, Kang SA, Park HY, Hong SL, Park HK, et al. Adipose-derived stem cells ameliorate allergic airway inflammation by inducing regulatory T cells in a mouse model of asthma. Mediators Inflamm. 2014; 2014:436476.

35. English K, Ryan JM, Tobin L, Murphy MJ, Barry FP, Mahon BP. Cell contact, prostaglandin E(2) and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+ CD25 (High) forkhead box P3+ regulatory T cells. Clin Exp Immunol. 2009; 156:149–60.
36. Nemeth K, Keane-Myers A, Brown JM, Metcalfe DD, Gorham JD, Bun-doc VG, et al. Bone marrow stromal cells use TGF-beta to suppress allergic responses in a mouse model of ragweed-induced asthma. Proc Natl Acad Sci U S A. 2010; 107:5652–7.
37. Cho KS, Lee JH, Park MK, Park HK, Yu HS, Roh HJ. Prostaglandin E2 and Transforming Growth Factor-β Play a Critical Role in Suppression of Allergic Airway Inflammation by Adipose-Derived Stem Cells. PLoS One. 2015; 10:e0131813.

Go to : 
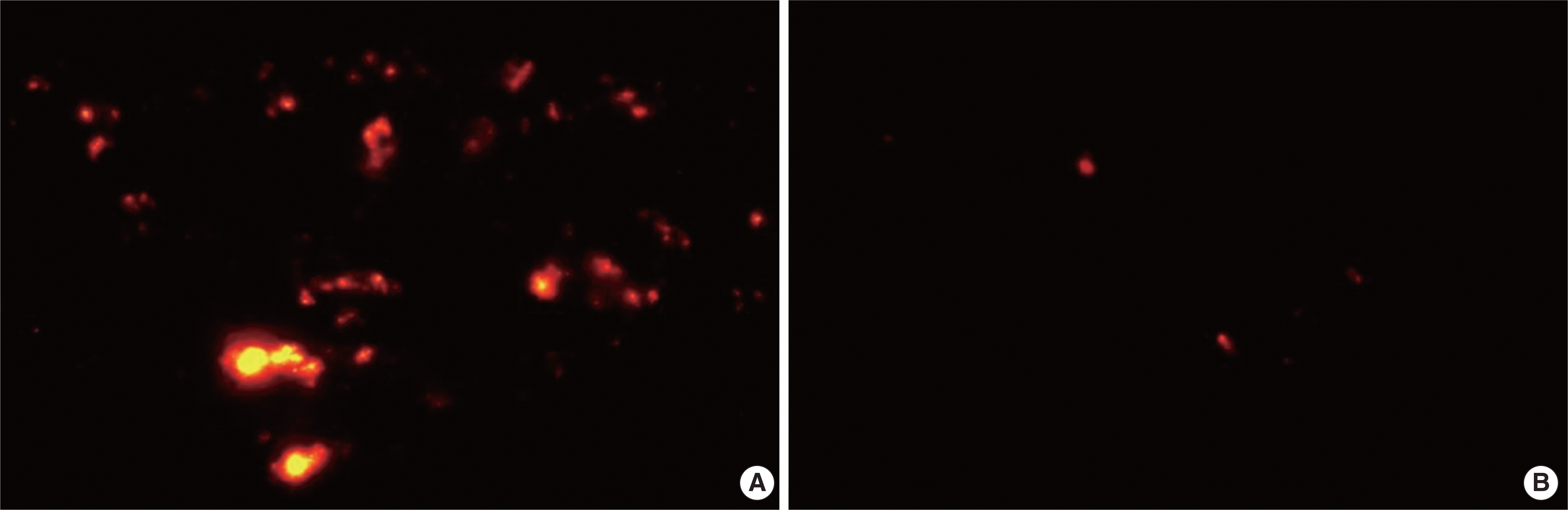 | Fig. 1.Adipose tissue-derived stem cells labeled with Cell Tracker CM-Dil (red) were more intensively distributed within the lung of asthmatic mice (A) than control mice (B) (magnification, ×200). |
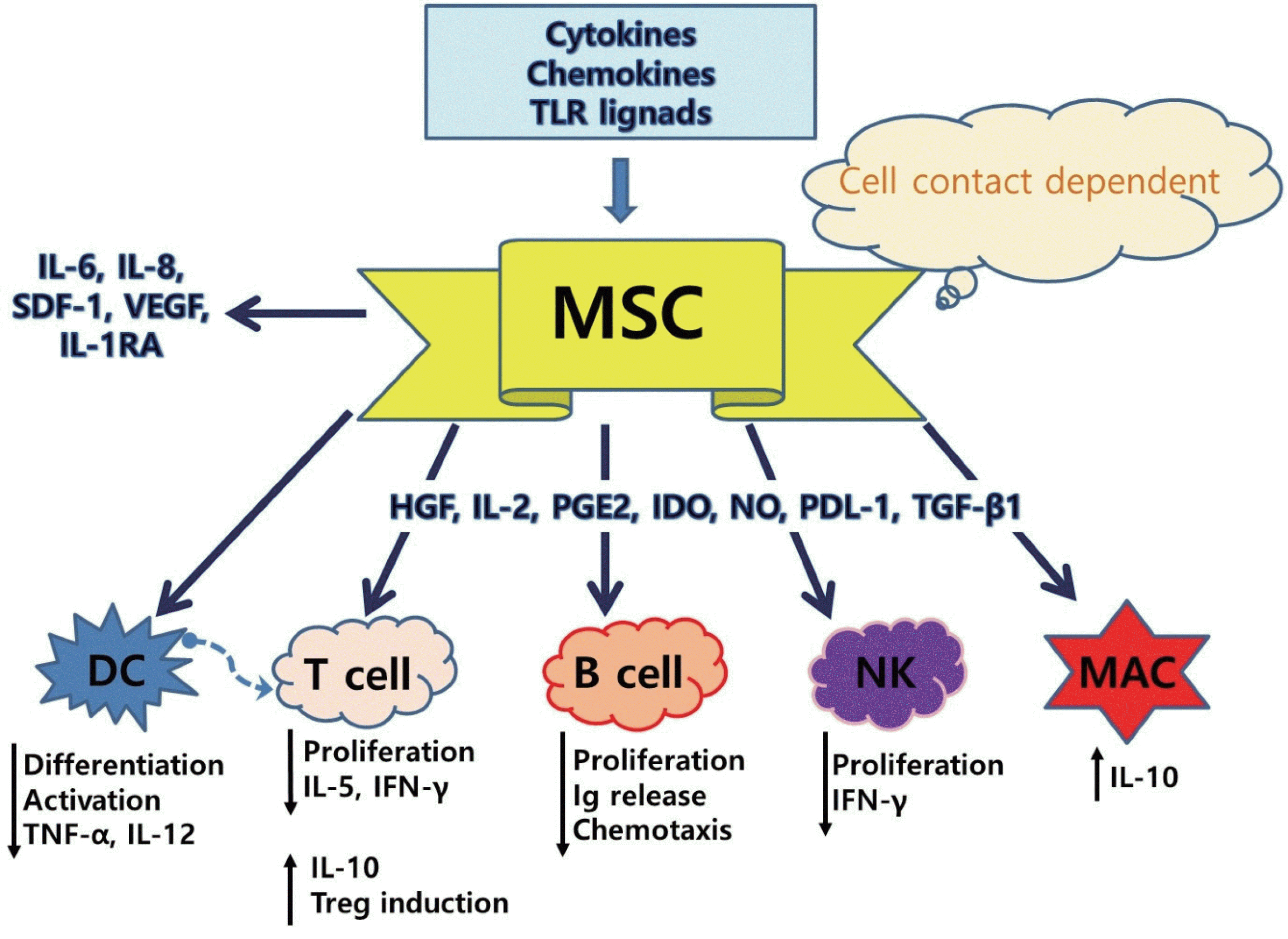 | Fig. 2.Schematic illustration of the soluble factors for MSC-mediated immunosuppression. TLR, toll-like receptor; MSC, mesenchymal stem cell; DC, dendritic cells; HGF, hepatocyte growth factor; IL, interleukin; PGE2, prostaglandin E2; IDO, indoleamine 2,3-dioxygenase; NO, nitric oxide; PDL-1, programmed death ligand-1; TGF-β1, transforming growth factor-β1; SDF-1, stem cell derived factor 1; VEGF, vascular endothelial growth factor; IL-1RA, interleukin-1 receptor antagonist; TNF-α, tumor necrosis factor-; IFN-, interferon; Ig, immunoglobulin; NK, natural killer; MAC, macrophage. Adapted from Sueblinvong et al. Transl Res 2010;156:188–205.33
|
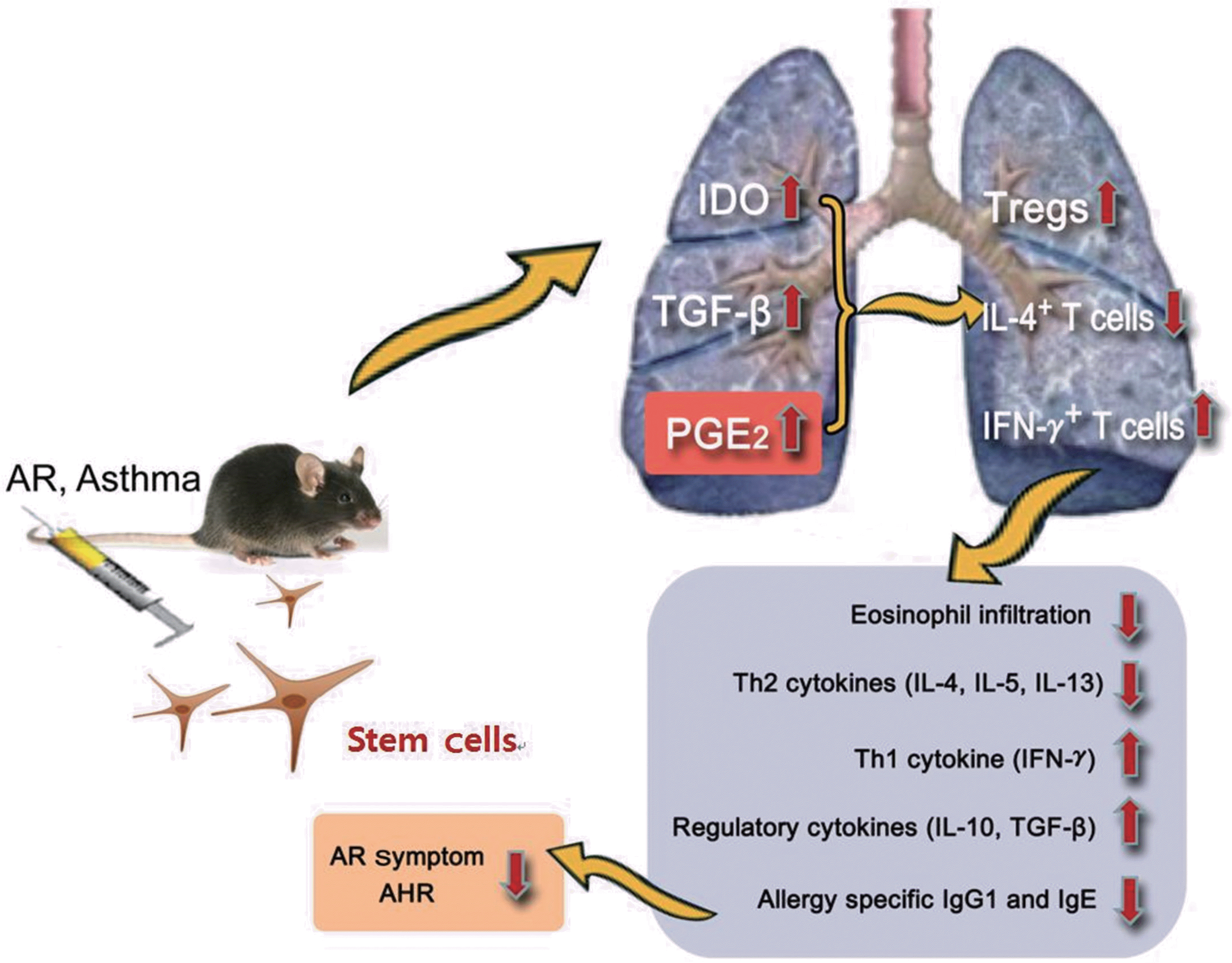 | Fig. 3.Schematic presentation of possible immunomodulatory mechanisms of stem cells in allergic airway diseases. AR, allergic rhinitis; AHR, airway hyperrespon-siveness; IDO, indoleamine 2,3-dioxygenase; IFN-γ, interferon-γ; Ig, immunoglobulin; IL, interleukin; PGE2, prostaglandin E2; TGF-β, transforming growth factor-β; Tregs, regulatory T cells. |
Table 1.
Summary of soluble factors critical for mesenchymal stem cells-medi-ated immunosuppression
iNOS, inducible nitric oxide synthase; CCL2, chemokine ligand 2; IDO, indoleamine 2,3-dioxygenase; NK, natural killer; HLA-G, human leukocyte antigen G; LIF, leukemia inhibitory factor; PBMC, peripheral blood mononuclear cells; TSG6, TNF-α stimulated gene/protein 6; HO-1, heme oxygenase-1; TGF-β, transforming growth factor-β; IL, interleukin; PGE2, prostaglandin E2; DC, dendritic cells; PD-L1/2, programmed cell death 1 ligand1/2; FasL, Fas ligand.
Table 2.
Proteins detected in the mesenchymal stem cell secretome




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download