Abstract
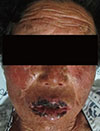
Stevens-Johnson syndrome (SJS) manifests with severe cutaneous reactions, most commonly triggered by medications, which are characterized by fever and mucocutaneous lesions leading to necrosis and sloughing of the epidermis. To our knowledge, pravastatin-induced SJS has not yet been reported. Here, we describe a case of SJS due to pravastatin, which was diagnosed by a patch test. A 70-year-old woman presented with maculopapular skin rashes, which developed 2 weeks after medication of bisoprolol, amlodipine, pravastatin, spironolactone, and indobufene for cardiac problems. Various bullous-erosive mucocutaneous lesions occupied less than 10% of the total body surface area. Painful oropharyngeal mucous membrane lesions were observed. The vermilion border of the lips became denuded and developed serosanguinous crusts. With the drug withdrawal and the use of systemic corticosteroids, her manifestations resolved. Drug patch tests with bisoprolol, amlodipine, pravastatin, spironolactone, and indobufene were performed, resulting in a positive reaction to pravastatin, but not to the other drugs. To the best of our knowledge, this is the first case of pravastatin-induced SJS.
Figures and Tables
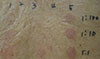 | Fig. 2Results of patch tests with spironolactone (1), bisoprolol (2), indobufene (3), amlodipine (4), and pravastatin (5) at the concentration of 1:100, 1:10, and 1:1 dilutions. 1:1 dilution denotes 25 mg/mL for spironolactone, 2.5 mg/mL for bisoprolol, 200 mg/mL for indobufene, 10 mg/mL for amlodipine, and 5 mg/mL for pravastatin. |
References
1. Fregert S. International Contact Dermatitis Research Group; North American Contact Dermatitis Group. Manual of contact dermatitis. 2nd ed. Copenhagen: Munksgaard Publishers;1981.
2. Peters AT, Peters NT. Erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis. In : Grammer LC, Greenberger PA, editors. Patterson's allergic diseases. 7th ed. 2009. p. 232–235.
3. Tas S, Simonart T. Management of drug rash with eosinophilia and systemic symptoms (DRESS syndrome): an update. Dermatology. 2003; 206:353–356.

4. Wolf R, Davidovici B, Matz H, Mahlab K, Orion E, Sthoeger ZM. Drug Rash with eosinophilia and systemic symptoms versus Stevens-Johnson Syndrome: a case that indicates a stumbling block in the current classification. Int Arch Allergy Immunol. 2006; 141:308–310.

5. Wolkenstein P, Chosidow O, Flechet ML, Robbiola O, Paul M, Dume L, et al. Patch testing in severe cutaneous adverse drug reactions, including Stevens-Johnson syndrome and toxic epidermal necrolysis. Contact Dermatitis. 1996; 35:234–236.

6. Hydzik P, Szpak D. Side effects of the HMG-CoA reductase inhibitors (statins). Lupus erythematosus induced by Atorvastatin therapy. Przegl Lek. 2011; 68:495–498.
7. Adams AE, Bobrove AM, Gilliam AC. Statins and "chameleon-like" cutaneous eruptions: simvastatin-induced acral cutaneous vesiculobullous and pustular eruption in a 70-year-old man. J Cutan Med Surg. 2010; 14:207–211.

8. Petrou E, Karali V, Papadakis E. Simvastatin-induced toxic epidermal necrolysis. J Acute Dis. 2014; 335–336.

9. Gressier L, Pruvost-Balland C, Dubertret L, Viguier M. Atorvastatin-induced drug reaction with eosinophilia and systemic symptoms (DRESS). Ann Dermatol Venereol. 2009; 136:50–53.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download