This article has been corrected. See "ERRATUM: Acknowledgments Correction. Iodinated contrast media-induced fixed drug eruption" in Volume 3 on page 461.
Abstract
Iodinated contrast media (ICM) can cause not only immediate onset hypersensitivity but also delayed onset hypersensitivity. While the most common form of delayed onset hypersensitivity reaction to ICM is exanthematous eruption, fixed drug eruption (FDE) can occur rarely related to ICM. A 70-year-old male with liver cirrhosis and hepatocellular carcinoma repeatedly experienced erythematous patches on his right forearm and hand 6 hours after exposure to iopromide for computed tomography scan. ICM induced FDE was diagnosed clinically. Intradermal test with 6 kinds of ICM (iobitridol, iohexol, iomeprol, iopamidol, iopromide, and iodixanol) was performed and showed the weakest positive reaction to iohexol compared to the others in 48 hours. After changing iopromide to iohexol based on these results, FDE did not recur. We report here a case of iopromide induced FDE which was successfully prevented by changing ICM to iohexol based on intradermal test results.
Figures and Tables
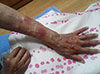 | Fig. 1Erythematous patch on the right forearm developed one day after computed tomography scan with iodinated contrast media. |
Table 1
The result of intradermal skin test with iodinated contrast media

| Purplish discoloration (mm) | |
|---|---|
| Saline | No increase |
| Iobitridol | 3.0 × 3.0 |
| Iohexol | 2.0 × 1.5 |
| Iomeprol | 3.0 × 3.0 |
| Iopamidol | 3.0 × 3.0 |
| Iopromide | 4.0 × 3.0 |
| Iodixanol | 3.0 × 3.0 |
Table 2
Summary of ICM induced fixed drug eruption case reports

| Source | Age/sex | Causative ICM | The clinical indication of ICM | Clinical features | Patch testing | Intradermal testing | Clinical courses |
|---|---|---|---|---|---|---|---|
| Wright and Cohen12) | 41 yr/F | Iopamidol | Underlying lupus nephritis, to evaluate arteriovenous shunt patency | Multiple; erythematous patches (3 cm×5 cm); the lower limbs; a few hours after administration | Iopamidol (+) | Not tested | Not stated |
| Iohexol (-) | |||||||
| Ioxagllic acid (-) | |||||||
| Iotrolan (-) | |||||||
| Iodoxamic acid (-) | |||||||
| Iodamid (-) | |||||||
| Ioversol (-) | |||||||
| Cha et al.3) | 69 yr/M | Iopromide | Underlying non-ST-elevation myocardial infarction, for percutaneous transluminal angioplasty | Multiple; erythematous patches (3-5 cm); both palms and trunk; several days after administration | Iopromide (-) | Not tested | Not stated |
| Benson et al.7) | 61 yr/M | Iopromide | To exclude the neoplasm | Single; erythematous patch (15 cm×8 cm); the right inguinal region; 4 hr after administration | Iopromide (-) | Not tested | After changing to iopamidol based on skin test, FDE did not recur. |
| Iomeprol (+) | |||||||
| Iohexol (+) | |||||||
| Iopamidol (-) | |||||||
| Iotrolan (-) | |||||||
| Iodixanol (-) | |||||||
| Ioxaglate (-) | |||||||
| Le Beller et al.10) | 67 yr/F | Iomeprol | To evaluate response for chemotherapy regularly (breast cancer) | Multiple; erythematous papules (pea-sized); the trunk and extremities; 4 days after administration | Iomeprol (+) | Iomeprol (+) | Not stated |
| Ioversol (-) | |||||||
| Iohexol (-) | |||||||
| Iotrolan (-) | |||||||
| Iopamidol (-) | |||||||
| Bohm et al.8) | 60 yr/M | Ioversol | To evaluate a aortic aneurysm regularly | Multiple; erythematous and blistering eruptions (1 cm); the back and both hands; 24 hr after administration | Ioversol (+) | Not tested | Not stated |
| Iopamidol (+) | |||||||
| Iohexol (+) | |||||||
| Hosoya et al.6) | 51 yr/F | Iothalamate | To evaluate a right-sided pulmonary mass | Single; erythematous plaque (8 cm); the right thigh; 12 hr after administration | Not tested | Not tested | Not stated |
| Watanabe et al.11) | 57 yr/F | Iohexol | Follow-up surveillance appointments for renal cell carcinoma every 6 months | Single; erythematous patch (9 cm×13 cm); the left breast; 15 hr after administration | Not tested | Not tested | Not stated |
| Frias et al.9) | 27 yr/F | Iodinaxol | Underlying end-stage renal failure due to AA amyloidosis, for arteriovenous fistula patency intervention | Multiple; erythematous lesions (size not clearly stated.); a few hours after administration | Not tested | Not tested | After changing to iobitridol empirically, FDE did not recur |
| Jin et al.14) | 48 yr/M | Iodinaxol | Underlying end-stage renal failures, for percutaneous transluminal angioplasty to arteriovenous fistula | Multiple; oval shaped, brownish to violaceous patches (size not clearly stated.); several hours after administration | Iopamido (-) | Not tested | Skin prick tests were performed, the results were all negative. |
| Iomeprol (-) | Provocation test was performed, the result was positive. | ||||||
| Ioversol (-) | |||||||
| Iopromide (-) | |||||||
| Iohexol (-) | |||||||
| Iodixanol (-) |
References
1. Shin MJ, Cho YJ. Management of adverse reaction to iodinated radiocontrast media. J Korean Med Assoc. 2012; 55:779–790.

2. Jung JW, Cho SH, Kim KH, Min KU, Kang HR. Clinical features of fixed drug eruption at a tertiary hospital in Korea. Allergy Asthma Immunol Res. 2014; 6:415–420.

3. Cha SH, Kim HS, Lee JY, Kim HO, Park YM. Fixed drug eruption due to iopromide (Ultravist). Ann Dermatol. 2011; 23:Suppl 1. S33–S35.

4. Kim MH, Lee SY, Lee SE, Kim MY, Jo EJ, Park CM, et al. Clinical features of delayed contrast media hypersensitivity. Allergy Asthma Respir Dis. 2014; 2:352–357.

5. Shiohara T, Mizukawa Y. Fixed drug eruption: the dark side of activation of intraepidermal CD8+ T cells uniquely specialized to mediate protective immunity. Chem Immunol Allergy. 2012; 97:106–121.

6. Hosoya T, Yamaguchi K, Akutsu T, Mitsuhashi Y, Kondo S, Sugai Y, et al. Delayed adverse reactions to iodinated contrast media and their risk factors. Radiat Med. 2000; 18:39–45.
7. Benson PM, Giblin WJ, Douglas DM. Transient, nonpigmenting fixed drug eruption caused by radiopaque contrast media. J Am Acad Dermatol. 1990; 23(2 Pt 2):379–381.

8. Bohm I, Medina J, Prieto P, Block W, Schild HH. Fixed drug eruption induced by an iodinated non-ionic X-ray contrast medium: a practical approach to identify the causative agent and to prevent its recurrence. Eur Radiol. 2007; 17:485–489.

9. Frias M, Fernandez E, Audicana MT, Longo N, Munoz D, Reyes SM. Fixed drug eruption caused by iodinated contrast media. Contact Dermatitis. 2011; 65:43–44.

10. Le Beller C, Fraitag S, Jacquot C, Lillo-Le Louet A, Auffret N. Fixed drug eruption caused by iodixanol. Ann Dermatol Venereol. 2008; 135:684–685.
11. Watanabe H, Sueki H, Nakada T, Akiyama M, Iijima M. Multiple fixed drug eruption caused by iomeprol (Iomeron), a nonionic contrast medium. Dermatology. 1999; 198:291–294.

12. Wright NA, Cohen PR. Fixed drug eruption associated with intravenous contrast media: report in a woman receiving iohexol. J Drugs Dermatol. 2011; 10:802–804.
13. Yamauchi R, Morita A, Tsuji T. Fixed drug eruption caused by iopamidol, a contrast medium. J Dermatol. 1997; 24:243–245.

14. Jin SY, Kim DH, Choi YS, Kwon JH, Lee AY, Lee SH. Iodixanol-induced fixed drug eruption diagnosed by systemic provocation test. Korean J Dermatol. 2012; 50:1073–1076.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download