Abstract
Purpose
Chlamydia pneumoniae is a common intracellular bacterial pathogen and plays an important role in acute respiratory infections. The purpose of this study was to investigate clinical presentations of C. pneumoniae in children with acute respiratory infections.
Methods
We examined the medical records of pediatric patients (age<18 years) admitted with acute respiratory infections of C. pneumoniae to Gachon University Gil Medical Center between March 1, 2011 and August 31, 2014. We compared the clinical features of C. pneumoniae infection with that of Mycoplasma pneumoniae infection.
Results
We confirmed acute respiratory infections of C. pneumoniae in 110 patients out of 2,156 patients (5.1%) admitted with acute respiratory infections. The mean age was 37.2±30.1 months. More than half of them (54.5%) had coinfection. C. pneumoniae infection had mild and subacute courses. The mean duration of symptoms prior to admission was 8.5±13.8 days. There were remarkable seasonal variations and prevalence was higher in December and April (P=0.03 and P=0.02, respectively). Although rhinorrhea and pharyngeal injection were more common in C. pneumoniae infection (P<0.05), clinical signs and symptoms were similar between C. pneumoniae and M. pneumoniae. Extrapulmonary manifestations such as skin lesion, Gastrointestinal symptoms, hepatitis, and neurologic symptoms were common (41.0%) in C. pneumoniae infection and, had similar incidence in M. pneumoniae infection.
Conclusion
C. pneumoniae is an important infectious agent of acute respiratory infections in children. Clinical pictures of C. pneumoniae are similar to M. pneumoniae, even in extrapulmonary manifestations. C. pneumoniae should be taken into consideration in differential diagnosis of acute respiratory infection in children.
Figures and Tables
 | Fig. 1Frequencies of infections caused by Chlamydia pneumoniae among children hospitalized in individual months of the year. |
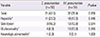
Table 1
Demographics of pediatric patients hospitalized with Chlamydia pneumoniae and Mycoplasma pneumoniae infections

Table 2
Clinical characteristics of hospitalized pediatric patients with Chlamydia pneumoniae and Mycoplasma pneumoniae infections
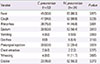
Table 3
Clinical courses of hospitalized pediatric patients with Chlamydia pneumoniae and Mycoplasma pneumoniae infections
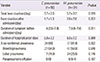
Table 4
Laboratory findings of hospitalized pediatric patients with Chlamydia pneumoniae and Mycoplasma pneumoniae infections
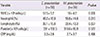
Table 5
Extrapulmonary manifestations of hospitalized pediatric patients with Chlamydia pneumoniae and Mycoplasma pneumoniae infections
References
1. Campbell LA, Kuo CC, Thissen RW, Grayston JT. Isolation of a gene encoding a Chlamydia sp. strain TWAR protein that is recognized during infection of humans. Infect Immun. 1989; 57:71–75.

2. Blasi F, Tarsia P, Arosio C, Fagetti L, Allegra L. Epidemiology of Chlamydia pneumoniae. Clin Microbiol Infect. 1998; 4:Suppl 4. S1–S6.

3. Yum HY, Choi JY, Rheu JW, Lee KE, Kim CH, Shon MH, et al. Correlation between Chlamydia pneumonia infection and childhood asthma. Pediatr Allergy Respir Dis. 2000; 10:218–224.
4. Grassi T, Mancini F, Ciervo A, Vescio MF, Ghazal A, Ashour H, et al. Chlamydophila pneumoniae, Mycoplasma pneumoniae, and influenza in children with respiratory infections in Alexandria, Egypt. J Infect Dev Ctries. 2014; 8:379–383.

5. Ishida T, Hashimoto T, Arita M, Ito I, Osawa M. Etiology of community-acquired pneumonia in hospitalized patients: a 3-year prospective study in Japan. Chest. 1998; 114:1588–1593.

6. Schmidt SM, Muller CE, Mahner B, Wiersbitzky SK. Prevalence, rate of persistence and respiratory tract symptoms of Chlamydia pneumoniae infection in 1211 kindergarten and school age children. Pediatr Infect Dis J. 2002; 21:758–762.

7. Schmidt SM, Muller CE, Krechting M, Wiersbitzky H, Gurtler L, Wiersbitzky SK. Chlamydia pneumoniae carriage and infection in hospitalized children with respiratory tract diseases. Infection. 2003; 31:410–416.

8. Chen Z, Ji W, Wang Y, Yan Y, Zhu H, Shao X, et al. Epidemiology and associations with climatic conditions of Mycoplasma pneumoniae and Chlamydophila pneumoniae infections among Chinese children hospitalized with acute respiratory infections. Ital J Pediatr. 2013; 39:34.

9. Esposito S, Blasi F, Bellini F, Allegra L, Principi N. Mowgli Study Group. Mycoplasma pneumoniae and Chlamydia pneumoniae infections in children with pneumonia. Mowgli Study Group. Eur Respir J. 2001; 17:241–245.

11. Imashuku S, Kudo N. Chlamydia pneumoniae infection-associated erythema multiforme. Pediatr Rep. 2013; 5:35–37.

12. Cunha BA, Pherez FM. C. pneumoniae community-acquired pneumonia (CAP) in mimicking Mycoplasma pneumoniae meningoencephalitis complicated by asthma. Heart Lung. 2009; 38:530–533.

13. Kuo CC, Jackson LA, Campbell LA, Grayston JT. Chlamydia pneumoniae (TWAR). Clin Microbiol Rev. 1995; 8:451–461.

14. Kanamoto Y, Ouchi K, Mizui M, Ushio M, Usui T. Prevalence of antibody to Chlamydia pneumoniae TWAR in japan. J Clin Microbiol. 1991; 29:816–818.

15. Lee HS, Chun BY, Jin SH, Lee WK. Infection rate of Chlamydia pneumoniae by serological antibody test between patients with respiratory symptoms and control group. Korean J Clin Microbiol. 2004; 7:31–37.
16. Miyashita N, Fukano H, Okimoto N, Hara H, Yoshida K, Niki Y, et al. Clinical presentation of community-acquired Chlamydia pneumoniae pneumonia in adults. Chest. 2002; 121:1776–1781.

17. Kauppinen MT, Saikku P, Kujala P, Herva E, Syrjala H. Clinical picture of community-acquired Chlamydia pneumoniae pneumonia requiring hospital treatment: a comparison between chlamydial and pneumococcal pneumonia. Thorax. 1996; 51:185–189.

18. File TM Jr, Plouffe JF Jr, Breiman RF, Skelton SK. Clinical characteristics of Chlamydia pneumoniae infection as the sole cause of community-acquired pneumonia. Clin Infect Dis. 1999; 29:426–428.

19. Miller ST, Hammerschlag MR, Chirgwin K, Rao SP, Roblin P, Gelling M, et al. Role of Chlamydia pneumoniae in acute chest syndrome of sickle cell disease. J Pediatr. 1991; 118:30–33.

20. Reechaipichitkul W, Saelee R, Lulitanond V. Prevalence and clinical features of Chlamydia pneumoniae pneumonia at Srinagarind Hospital, Khon Kaen, Thailand. Southeast Asian J Trop Med Public Health. 2005; 36:151–155.
21. Ngeow YF, Suwanjutha S, Chantarojanasriri T, Wang F, Saniel M, Alejandria M, et al. An Asian study on the prevalence of atypical respiratory pathogens in community-acquired pneumonia. Int J Infect Dis. 2005; 9:144–153.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download