Abstract
Figures and Tables
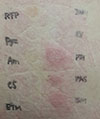 | Fig. 1Skin findings of patch test to antitubercular drugs read at 48 hours. Test drugs are isoniazid (INH), levofloxacin (LV), prothinamide (PTH), p-aminosalicylic acid (PAS), streptomycin (SM), ethambutol (ETM), cycloserine (CS), amoxicillin clavulanate (Am), pyrazinamide (Pyz), rifampicin (RFP) in clockwise direction from the right top. |
Table 1
Summary of drug administration and related symptoms

● , administration of full dose; □ , discontinuation; ▲ , administration with desensitization protocol; △ , hypersensitivity reaction despite administration with desensitization protocol; PZA, pyrazinamide; SM, streptomycin; CS, cycloserine; PAS, p-aminosalicylic acid; PTH, prothionamide; LVFX, levofloxacin; KM, kanamycin; AC, amoxicillin clavulanate.
Table 2
Intravenous rapid desensitization protocol for kanamycin (target dose: 1,000 mg)
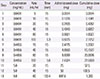
Table 3
Oral rapid desensitization protocol for prothionamide (target dose: 250 mg twice a day)
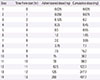
Table 4
Oral rapid desensitization protocol for cycloserine (target dose: 500 mg twice a day)




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download