Abstract
Cetuximab, a chimeric mouse-human immunoglobulin, is an antiepidermal growth factor receptor monoclonal antibody. It has been approved by the U.S. Food and Drug Administration for the treatment of metastatic colorectal and head/neck cancer, but can cause fatal hypersensitivity reactions in some patients. A 66-year-old male with metastatic sigmoid cancer had cetuximab-induced anaphylaxis when the first dose of cetuximab was administered. Cetuximab was safely readministered for another 15 cycles based on the rapid desensitization protocol. We experienced a case of cetuximab-induced anaphylaxis on the first exposure which was successfully managed by rapid desensitization.
Figures and Tables
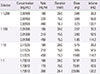
Table 1
The preparation of 4-bottle tenfold increasing solutions used in the rapid 12-step desensitization protocol in the present case of the cetuximab-induced delayed hypersensitivity
References
1. Sampson HA, Munoz-Furlong A, Campbell RL, Adkinson NF Jr, Bock SA, Branum A, et al. Second symposium on the definition and management of anaphylaxis: summary report--second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. Ann Emerg Med. 2006; 47:373–380.

2. Wood RA, Camargo CA Jr, Lieberman P, Sampson HA, Schwartz LB, Zitt M, et al. Anaphylaxis in America: the prevalence and characteristics of anaphylaxis in the United States. J Allergy Clin Immunol. 2014; 133:461–467.

3. Lee SY, Yang MS, Jung JW, Oh MJ, Park CH, Sohn SW, et al. Updates on desensitization for hypersensitivity reactions related to chemotherapy. Allergy Asthma Respir Dis. 2013; 1:295–302.

4. Zanotti KM, Markman M. Prevention and management of antineoplastic-induced hypersensitivity reactions. Drug Saf. 2001; 24:767–779.

5. Jerath MR, Kwan M, Kannarkat M, Mirakhur B, Carey L, Valgus J, et al. A desensitization protocol for the mAb cetuximab. J Allergy Clin Immunol. 2009; 123:260–262.

6. Castells MC, Tennant NM, Sloane DE, Hsu FI, Barrett NA, Hong DI, et al. Hypersensitivity reactions to chemotherapy: outcomes and safety of rapid desensitization in 413 cases. J Allergy Clin Immunol. 2008; 122:574–580.

7. Madrigal-Burgaleta R, Berges-Gimeno MP, Angel-Pereira D, Ferreiro-Monteagudo R, Guillen-Ponce C, Pueyo C, et al. Hypersensitivity and desensitization to antineoplastic agents: outcomes of 189 procedures with a new short protocol and novel diagnostic tools assessment. Allergy. 2013; 68:853–861.

8. Graham J, Muhsin M, Kirkpatrick P. Fresh from the pipeline: cetuximab. Nat Rev Drug Discov. 2004; 3:549.
9. Van Cutsem E, Kohne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009; 360:1408–1417.

10. Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008; 359:1116–1127.

11. Kang SP, Saif MW. Infusion-related and hypersensitivity reactions of monoclonal antibodies used to treat colorectal cancer--identification, prevention, and management. J Support Oncol. 2007; 5:451–457.
12. Needle MN. Safety experience with IMC-C225, an anti-epidermal growth factor receptor antibody. Semin oncol. 2002; 29:5 Suppl 14. 55–60.

13. Chung CH, Mirakhur B, Chan E, Le QT, Berlin J, Morse M, et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N Engl J Med. 2008; 358:1109–1117.

14. Lee JH, Kim JH, Kim TH, Kim SC. Delayed mammalian meat-induced anaphylaxis confirmed by skin test to cetuximab. J Dermatol. 2013; 40:577–578.

15. Naclerio R, Mizrahi EA, Adkinson NF Jr. Immunologic observations during desensitization and maintenance of clinical tolerance to penicillin. J Allergy Clin Immunol. 1983; 71:294–301.

16. Kepley CL. Antigen-induced reduction in mast cell and basophil functional responses due to reduced Syk protein levels. Int Arch Allergy Immunol. 2005; 138:29–39.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download