Abstract
Purpose
L-asparaginase is a crucial chemotherapeutic agent for the treatment of acute lymphoblastic leukemia. However, hypersensitivity to L-asparaginase is common which limits its clinical use.
Methods
We performed 44 cases of premedication and 3 cases of desensitization in 16 patients with hypersensitivity to L-asparaginase.
Results
With premedication, 33 cases completed L-asparaginase injection with no hypersensitivity reactions. Eleven cases showed mild hypersensitivity reactions, such as urticaria. Desensitization was performed in 3 cases: in 2 cases, desensitization was successful, and in 1 case the medication was switched to Erwinia asparaginase.
Figures and Tables
Table 1
Definition of adverse event according to common terminology criteria for adverse event

Table 2A
Example of desensitization protocol for asparaginase (7,000 IU)
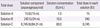
Table 2B
Stepwise doubling of doses for asparaginase (7,000 IU)
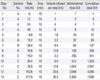
Table 3
Characteristics of study population (n=16)

Table 4
Clinical and laboratory characteristics of the patients with L-asparaginase hypersensitivity
References
3. Woo MH, Hak LJ, Storm MC, Evans WE, Sandlund JT, Rivera GK, et al. Anti-asparaginase antibodies following E. coli asparaginase therapy in pediatric acute lymphoblastic leukemia. Leukemia. 1998; 12:1527–1533.

4. Narta UK, Kanwar SS, Azmi W. Pharmacological and clinical evaluation of L-asparaginase in the treatment of leukemia. Crit Rev Oncol Hematol. 2007; 61:208–221.

5. Pratt CB, Simone JV, Zee P, Aur RJ, Johnson WW. Comparison of daily versus weekly L-asparaginase for the treatment of childhood acute leukemia. J Pediatr. 1970; 77:474–483.

6. Cheung NK, Chau IY, Coccia PF. Antibody response to Escherichia coli L-asparaginase. Prognostic significance and clinical utility of antibody measurement. Am J Pediatr Hematol Oncol. 1986; 8:99–104.
7. Pratt CB, Choi SI, Holton CP. Low-dosage asparaginase treatment of childhood acute lymphocytic leukemia. Am J Dis Child. 1971; 121:406–409.

8. Haskell CM, Canellos GP. l-asparaginase resistance in human leukemia: asparagine synthetase. Biochem Pharmacol. 1969; 18:2578–2580.
9. Soyer OU, Aytac S, Tuncer A, Cetin M, Yetgin S, Sekerel BE. Alternative algorithm for L-asparaginase allergy in children with acute lymphoblastic leukemia. J Allergy Clin Immunol. 2009; 123:895–899.

10. Hak LJ, Relling MV, Cheng C, Pei D, Wang B, Sandlund JT, et al. Asparaginase pharmacodynamics differ by formulation among children with newly diagnosed acute lymphoblastic leukemia. Leukemia. 2004; 18:1072–1077.

11. Wang B, Relling MV, Storm MC, Woo MH, Ribeiro R, Pui CH, et al. Evaluation of immunologic crossreaction of antiasparaginase antibodies in acute lymphoblastic leukemia (ALL) and lymphoma patients. Leukemia. 2003; 17:1583–1588.

12. Castells MC, Tennant NM, Sloane DE, Hsu FI, Barrett NA, Hong DI, et al. Hypersensitivity reactions to chemotherapy: outcomes and safety of rapid desensitization in 413 cases. J Allergy Clin Immunol. 2008; 122:574–580.

13. Bonno M, Kawasaki H, Hori H, Umemoto M, Komada Y, Sakurai M. Rapid desensitization for L-asparaginase hypersensitivity. J Allergy Clin Immunol. 1998; 101(4 Pt 1):571–572.

14. Sampson HA, Munoz-Furlong A, Campbell RL, Adkinson NF Jr, Bock SA, Branum A, et al. Second symposium on the definition and management of anaphylaxis: summary report: Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006; 117:391–397.

15. Common terminology criteriafor adverse events (CTCAE). Version 4.0 [Internet]. Bethesda (MD): U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute;c2010. cited 2014 Aug 3. Available from: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf.
16. Johansson SG, Hourihane JO, Bousquet J, Bruijnzeel-Koomen C, Dreborg S, Haahtela T, et al. A revised nomenclature for allergy. An EAACI position statement from the EAACI nomenclature task force. Allergy. 2001; 56:813–824.

18. Limsuwan T, Castells MC. Outcomes and safety of rapid desensitization for chemotherapy hypersensitivity. Expert Opin Drug Saf. 2010; 9:39–53.

19. Tallal L, Tan C, Oettgen H, Wollner N, McCarthy M, Helson L, et al. E. coli L-asparaginase in the treatment of leukemia and solid tumors in 131 children. Cancer. 1970; 25:306–320.

20. Clarkson B, Krakoff I, Burchenal J, Karnofsky D, Golbey R, Dowling M, et al. Clinical results of treatment with E. coli L-asparaginase in adults with leukemia, lymphoma, and solid tumors. Cancer. 1970; 25:279–305.

21. Moghrabi A, Levy DE, Asselin B, Barr R, Clavell L, Hurwitz C, et al. Results of the Dana-Farber Cancer Institute ALL Consortium Protocol 95-01 for children with acute lymphoblastic leukemia. Blood. 2007; 109:896–904.

22. Silverman LB, Stevenson K, Neuberg D, O'Brien J, Supko J, Sallan SE. Intravenous PEG asparaginase during remission induction for childhood ALL [abstract]. Blood. 2006; 108:1854.

23. Raetz EA, Salzer WL. Tolerability and efficacy of L-asparaginase therapy in pediatric patients with acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2010; 32:554–563.

24. Billett AL, Carls A, Gelber RD, Sallan SE. Allergic reactions to Erwinia asparaginase in children with acute lymphoblastic leukemia who had previous allergic reactions to Escherichia coli asparaginase. Cancer. 1992; 70:201–206.

25. Salzer WL, Asselin B, Supko JG, Devidas M, Kaiser NA, Plourde P, et al. Erwinia asparaginase achieves therapeutic activity after pegaspargase allergy: a report from the Children's Oncology Group. Blood. 2013; 122:507–514.

26. Muller HJ, Boos J. Use of L-asparaginase in childhood ALL. Crit Rev Oncol Hematol. 1998; 28:97–113.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download