Abstract
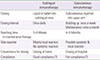
Allergen specific immunotherapy is a medical treatment aiming at patients suffering from allergies that are insufficiently controlled by symptomatic treatments. Allergen immunotherapy rehabilitates the immune system. Subcutaneous immunotherapy (SCIT) is the historical route of administration and consists of allergen extract injections. SCIT has proven efficacy in allergic rhinitis and asthma, but it requires regular injections at the hospital and carries the risk of potentially serious systemic allergic reactions in response to the treatment itself. Sublingual immunotherapy (SLIT) offers several specific advantages over SCIT. SLIT is more easily administered, avoids cumbersome injections regimens, and carries a much lower risk of anaphylactic shock compared with SCIT. So, this article will discuss the mechanisms of action, advantages, and limitations of SLIT for allergic rhinitis.
Figures and Tables
References
1. Togias A. Rhinitis and asthma: evidence for respiratory system integration. J Allergy Clin Immunol. 2003; 111:1171–1183.

2. Sur DK, Scandale S. Treatment of allergic rhinitis. Am Fam Physician. 2010; 81:1440–1446.
4. Cohen SG, Evans R 3rd. Allergen immunotherapy in historical perspective. Clin Allergy Immunol. 2004; 18:1–36.
5. Joint Task Force on Practice Parameters. American Academy of Allergy, Asthma and Immunology. American College of Allergy, Asthma and Immunology. Joint Council of Allergy, Asthma and Immunology. Allergen immunotherapy: a practice parameter second update. J Allergy Clin Immunol. 2007; 120:3 Suppl. S25–S85.
6. Scadding GK, Durham SR, Mirakian R, Jones NS, Leech SC, Farooque S, et al. BSACI guidelines for the management of allergic and non-allergic rhinitis. Clin Exp Allergy. 2008; 38:19–42.

7. Canonica GW, Bousquet J, Casale T, Lockey RF, Baena-Cagnani CE, Pawankar R, et al. Sub-lingual immunotherapy: world allergy organization position paper 2009. Allergy. 2009; 64:Suppl 91. 1–59.

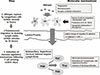
8. Moingeon P, Batard T, Fadel R, Frati F, Sieber J, Van Overtvelt L. Immune mechanisms of allergen-specific sublingual immunotherapy. Allergy. 2006; 61:151–165.

9. La Rosa M, Ranno C, Andre C, Carat F, Tosca MA, Canonica GW. Double-blind placebo-controlled evaluation of sublingual-swallow immunotherapy with standardized Parietaria judaica extract in children with allergic rhinoconjunctivitis. J Allergy Clin Immunol. 1999; 104(2 Pt 1):425–432.

10. Lima MT, Wilson D, Pitkin L, Roberts A, Nouri-Aria K, Jacobson M, et al. Grass pollen sublingual immunotherapy for seasonal rhinoconjunctivitis: a randomized controlled trial. Clin Exp Allergy. 2002; 32:507–514.

11. Tari MG, Mancino M, Monti G. Efficacy of sublingual immunotherapy in patients with rhinitis and asthma due to house dust mite: a double-blind study. Allergol Immunopathol (Madr). 1990; 18:277–284.
12. Smith H, White P, Annila I, Poole J, Andre C, Frew A. Randomized controlled trial of high-dose sublingual immunotherapy to treat seasonal allergic rhinitis. J Allergy Clin Immunol. 2004; 114:831–837.

13. Radulovic S, Wilson D, Calderon M, Durham S. Systematic reviews of sublingual immunotherapy (SLIT). Allergy. 2011; 66:740–752.

14. Wilson DR, Lima MT, Durham SR. Sublingual immunotherapy for allergic rhinitis: systematic review and meta-analysis. Allergy. 2005; 60:4–12.

15. Scadding G, Durham SR. Mechanisms of sublingual immunotherapy. Immunol Allergy Clin North Am. 2011; 31:191–209.

16. Pajno GB, Morabito L, Barberio G, Parmiani S. Clinical and immunologic effects of long-term sublingual immunotherapy in asthmatic children sensitized to mites: a double-blind, placebo-controlled study. Allergy. 2000; 55:842–849.

17. Kim ST, Han DH, Moon IJ, Lee CH, Min YG, Rhee CS. Clinical and immunologic effects of sublingual immunotherapy on patients with allergic rhinitis to house-dust mites: 1-year follow-up results. Am J Rhinol Allergy. 2010; 24:271–275.

18. Malling HJ, Lund L, Ipsen H, Poulsen L. Safety and immunological changes during sublingual immunotherapy with standardized quality grass allergen tablets. J Investig Allergol Clin Immunol. 2006; 16:162–168.
19. Bahceciler NN, Arikan C, Taylor A, Akdis M, Blaser K, Barlan IB, et al. Impact of sublingual immunotherapy on specific antibody levels in asthmatic children allergic to house dust mites. Int Arch Allergy Immunol. 2005; 136:287–294.

20. Ciprandi G, Cirillo I, Fenoglio D, Marseglia G, Tosca MA. Sublingual immunotherapy induces spirometric improvement associated with IL-10 production: preliminary reports. Int Immunopharmacol. 2006; 6:1370–1373.

21. Marcucci F, Sensi L, Frati F, Senna GE, Canonica GW, Parmiani S, et al. Sublingual tryptase and ECP in children treated with grass pollen sublingual immunotherapy (SLIT): safety and immunologic implications. Allergy. 2001; 56:1091–1095.

22. Passalacqua G, Durham SR. Global Allergy and Asthma European Network. Allergic rhinitis and its impact on asthma update: allergen immunotherapy. J Allergy Clin Immunol. 2007; 119:881–891.

23. Akdis M, Verhagen J, Taylor A, Karamloo F, Karagiannidis C, Crameri R, et al. Immune responses in healthy and allergic individuals are characterized by a fine balance between allergen-specific T regulatory 1 and T helper 2 cells. J Exp Med. 2004; 199:1567–1575.

24. Akdis CA, Blesken T, Akdis M, Wuthrich B, Blaser K. Role of interleukin 10 in specific immunotherapy. J Clin Invest. 1998; 102:98–106.

25. Jutel M, Akdis M, Budak F, Aebischer-Casaulta C, Wrzyszcz M, Blaser K, et al. IL-10 and TGF-beta cooperate in the regulatory T cell response to mucosal allergens in normal immunity and specific immunotherapy. Eur J Immunol. 2003; 33:1205–1214.

26. Arikan C, Bahceciler NN, Deniz G, Akdis M, Akkoc T, Akdis CA, et al. Bacillus Calmette-Guérin-induced interleukin-12 did not additionally improve clinical and immunologic parameters in asthmatic children treated with sublingual immunotherapy. Clin Exp Allergy. 2004; 34:398–405.

27. Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy. 2008; 63:Suppl 86. 8–160.
28. Wüthrich B, Bucher Ch, Jorg W, Bircher A, Eng P, Schneider Y, et al. Double-blind, placebo-controlled study with sublingual immunotherapy in children with seasonal allergic rhinitis to grass pollen. J Investig Allergol Clin Immunol. 2003; 13:145–148.
29. Olaguíbel JM, Alvarez Puebla MJ. Efficacy of sublingual allergen vaccination for respiratory allergy in children. Conclusions from one meta-analysis. J Investig Allergol Clin Immunol. 2005; 15:9–16.
30. Penagos M, Passalacqua G, Compalati E, Baena-Cagnani CE, Orozco S, Pedroza A, et al. Metaanalysis of the efficacy of sublingual immunotherapy in the treatment of allergic asthma in pediatric patients, 3 to 18 years of age. Chest. 2008; 133:599–609.

31. Di Bona D, Plaia A, Scafidi V, Leto-Barone MS, Di Lorenzo G. Efficacy of sublingual immunotherapy with grass allergens for seasonal allergic rhinitis: a systematic review and meta-analysis. J Allergy Clin Immunol. 2010; 126:558–566.

32. Bufe A, Ziegler-Kirbach E, Stoeckmann E, Heidemann P, Gehlhar K, Holland-Letz T, et al. Efficacy of sublingual swallow immunotherapy in children with severe grass pollen allergic symptoms: a double-blind placebo-controlled study. Allergy. 2004; 59:498–504.

33. Guez S, Vatrinet C, Fadel R, Andre C. House-dust-mite sublingual-swallow immunotherapy (SLIT) in perennial rhinitis: a double-blind, placebo-controlled study. Allergy. 2000; 55:369–375.

34. Durham SR, Yang WH, Pedersen MR, Johansen N, Rak S. Sublingual immunotherapy with once-daily grass allergen tablets: a randomized controlled trial in seasonal allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2006; 117:802–809.

35. Novembre E, Galli E, Landi F, Caffarelli C, Pifferi M, De Marco E, et al. Coseasonal sublingual immunotherapy reduces the development of asthma in children with allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2004; 114:851–857.

36. Marogna M, Tomassetti D, Bernasconi A, Colombo F, Massolo A, Businco AD, et al. Preventive effects of sublingual immunotherapy in childhood: an open randomized controlled study. Ann Allergy Asthma Immunol. 2008; 101:206–211.

37. Winther L, Malling HJ, Mosbech H. Allergen-specific immunotherapy in birch- and grass-pollen-allergic rhinitis. II. Side-effects. Allergy. 2000; 55:827–835.

38. Moreno C, Cuesta-Herranz J, Fernandez-Tavora L, Alvarez-Cuesta E. Immunotherapy Committee, Sociedad Espanola de Alergologia e Inmunologia Clinica. Immunotherapy safety: a prospective multi-centric monitoring study of biologically standardized therapeutic vaccines for allergic diseases. Clin Exp Allergy. 2004; 34:527–531.

39. Kleine-Tebbe J, Ribel M, Herold DA. Safety of a SQ-standardised grass allergen tablet for sublingual immunotherapy: a randomized, placebo-controlled trial. Allergy. 2006; 61:181–184.

40. Casale TB, Canonica GW, Bousquet J, Cox L, Lockey R, Nelson HS, et al. Recommendations for appropriate sublingual immunotherapy clinical trials. J Allergy Clin Immunol. 2009; 124:665–670.

41. Cox LS, Larenas Linnemann D, Nolte H, Weldon D, Finegold I, Nelson HS. Sublingual immunotherapy: a comprehensive review. J Allergy Clin Immunol. 2006; 117:1021–1035.

42. Bousquet J, Lockey R, Malling HJ. Allergen immunotherapy: therapeutic vaccines for allergic diseases. A WHO position paper. J Allergy Clin Immunol. 1998; 102(4 Pt 1):558–562.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download