Abstract
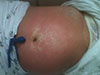
Cudrania tricuspidata is a deciduous tree belonging to the Moraceae plant, which has been widely used as a folk remedy or health supplements in the Asian countries including Korea. As far as we know, side effects from taking the extract of C. tricuspidata has not yet been reported. We reviewed the electronic medical records of 2 patients who had adverse drug reactions to C. tricuspidata. The first case was a 30-year-old woman without a specific medical history. She was admitted with a 2-week history of jaundice and dyspepsia after taking extract of C. tricuspidata for 3 days. Initial laboratory findings were as follows: aspartate aminotransferase, 364 IU/L; alanine aminotransferase, 574 IU/L; total bilirubin, 36.3 mg/dL; and direct bilirubin, 18.3 mg/dL. She was conservatively treated for liver and renal failure while awaiting liver transplantation. However, she was expired due to combined pneumonia and progressed hepatic and renal failure. The second case was a 42-year-old woman who has chronic urticaria without other medical history. She was admitted with a 3-month history of whole body rash with small pustular vesicle after taking extract of C. tricuspidata. She was treated with intravenous steroids and antihistamines. Skin lesions were improved after 1 week. Here, we report 2 cases of adverse drug reaction to C. tricuspidata. It should be considered that C. tricuspidata ingestion could cause severe adverse drug reactions such as liver failure and acute generalized exanthematous pustulosis.
Figures and Tables
References
1. Fujimoto T, Hano Y, Nomura T, Uzawa J. Components of root bark of Cudrania tricuspidata 2. Structures of two new isoprenylated flavones, Cudraflavones A and B. Planta Med. 1984; 50:161–163.

2. An RB, Sohn DH, Kim YC. Hepatoprotective compounds of the roots of Cudrania tricuspidata on tacrine-induced cytotoxicity in Hep G2 cells. Biol Pharm Bull. 2006; 29:838–840.

3. Hano Y, Matsumoto Y, Shinohara K, Sun JY, Nomura T. Structures of four new isoprenylated Xanthones, Cudraxanthones L, M, N, and O from Cudrania tricuspidata1, 2. Planta Med. 1991; 57:172–175.

4. Zou YS, Hou AJ, Zhu GF, Chen YF, Sun HD, Zhao QS. Cytotoxic isoprenylated xanthones from Cudrania tricuspidata. Bioorg Med Chem. 2004; 12:1947–1953.

5. Bent S, Ko R. Commonly used herbal medicines in the United States: a review. Am J Med. 2004; 116:478–485.

6. Choi SR, You DH, Kim JY, Park CB, Kim DH, Ryu J, et al. Antimicrobial activity of methanol extracts from Cudrania tricuspidata Bureau according to the parts harvested and time. Korean J Med Crop Sci. 2009; 17:335–340.
7. Ahn BM. Herbal preparation-induced liver injury. Korean J Gastroenterol. 2004; 44:113–125.
8. Cho JC, Lee HK, Choi JW, Lee YS, Jung YW, Seo DJ. A case of acute hepatitis related to the Chinese medicine Ho-Shou-Wu. Korean J Med. 1999; 56:753–756.
9. Lee JS, Lee DY, Kim YM, Lee JH, Ryu IY, Yun SJ, et al. Seventeen cases with herbal madicine-induced hepatitis. Korean J Gastroenterol. 1998; 32:69–74.
10. Chang SL, Huang YH, Yang CH, Hu S, Hong HS. Clinical manifestations and characteristics of patients with acute generalized exanthematous pustulosis in Asia. Acta Derm Venereol. 2008; 88:363–365.
11. Park YM, Park JG, Kang H, Houh D, Byun DG, Kim JW. Acute generalized exanthematous pustulosis induced by ingestion of lacquer chicken. Br J Dermatol. 2000; 143:230–232.

12. Sidoroff A, Dunant A, Viboud C, Halevy S, Bavinck JN, Naldi L, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP)-results of a multinational case-control study (EuroSCAR). Br J Dermatol. 2007; 157:989–996.

13. Park SJ, Lim YS, Hwang S, Heo NY, Lee HC, Suh DJ, et al. Emergency adult-to-adult living-donor liver transplantation for acute liver failure in a hepatitis B virus endemic area. Hepatology. 2010; 51:903–911.

14. Seo JC, Jeon WJ, Park SS, Kim SH, Lee KM, Chae HB, et al. Clinical experience of 48 acute toxic hepatitis patients. Korean J Hepatol. 2006; 12:74–81.
15. Nin Chau T, Cheung WI, Ngan T, Lin J, Lee KW, Tat Poon W, et al. Causality assessment of herb-induced liver injury using multidisciplinary approach and Roussel Uclaf Causality Assessment Method (RUCAM). Clin Toxicol (Phila). 2011; 49:34–39.

16. Taberner R, Puig L, Gilaberte M, Alomar A. Acute generalized exanthematous pustulosis induced by terbinafine. Eur J Dermatol. 2003; 13:313–314.
17. Choi MJ, Kim HS, Park HJ, Park CJ, Lee JD, Lee JY, et al. Clinicopathologic manifestations of 36 korean patients with acute generalized exanthematous pustulosis: a case series and review of the literature. Ann Dermatol. 2010; 22:163–169.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download