Abstract
Purpose
There has recently been increasing interest in the use of exhaled breath condensate (EBC) as a simple noninvasive means for understanding the physiology of asthma. The aim of this study was to evaluate the levels of leukotriene B4 (LTB4) and eosinophil cationic protein (ECP) in the EBC of asthmatic children.
Methods
We measured LTB4 and ECP levels in EBC from children aged 6-14 years, including healthy children (n=25) and asthmatic children (n=25). We also measured serum LTB4 and serum ECP. Pulmonary function tests and methacholine challenge tests were performed on all subjects.
Results
Exhaled LTB4 levels were increased significantly in patients with asthma compared to normal subjects (7.1±3.7 pg/mL vs. 2.2±1.7 pg/mL, P<0.05). Serum LTB4 levels were not significantly different in patients with asthma compared to normal subjects (674.7±484.1 pg/mL vs. 487.1±272.0 pg/mL, P=0.156,) and no significant correlations were found between exhaled and serum LTB4 concentrations in children with asthma (r=0.052, P=0.758). Exhaled ECP levels were not significantly different in patients with asthma compared to normal subjects (P=0.419). Serum ECP levels were significantly increased in patients with asthma compared to normal subjects (44.37±32.14 µg/L vs. 16.40±13.23 µg/L, P=0.001).
Figures and Tables
Fig. 1
(A) Comparison of concentrations of leukotriene B4 (LTB4) in exhaled breath condensate between asthma and control groups. LTB4 in asthma group (mean±standard deviation [SD], 7.1±3.7 pg/mL) was significantly higher than control group (mean±SD, 2.2±1.7 pg/mL) (P=0.03). (B) Comparison of concentrations of serum LTB4 between asthma and control groups. There was no significant difference between two groups (P=0.156).
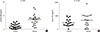
Fig. 2
Correlations between the concentrations of leukotriene B4 (LTB4) in exhaled breath condensate (EBC) and in serum. No significant correlations were shown between concentrations of LTB4 in EBC and in serum (r=0.052, P=0.758).
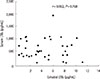
Fig. 3
(A) Comparison of concentrations of eosinophil cationic protein (ECP) in exhaled breath condensate (EBC) between asthma and control groups. There was no significant difference between two groups (P=0.419). (B) Comparison of concentrations of serum ECP between asthma and control groups. Serum concentration of ECP in asthma group (mean±standard deviation [SD], 44.37±32.14 µg/L) was significantly higher than control group (mean±SD, 16.40±13.23 µg/L) (P=0.01).
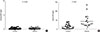
References
1. Hunt J. Exhaled breath condensate: an evolving tool for noninvasive evaluation of lung disease. J Allergy Clin Immunol. 2002; 110:28–34.

2. Rosias PP, Dompeling E, Dentener MA, Pennings HJ, Hendriks HJ, Van Iersel MP, et al. Childhood asthma: exhaled markers of airway inflammation, asthma control score, and lung function tests. Pediatr Pulmonol. 2004; 38:107–114.

3. Leung TF, Wong GW, Ko FW, Li CY, Yung E, Lam CW, et al. Analysis of growth factors and inflammatory cytokines in exhaled breath condensate from asthmatic children. Int Arch Allergy Immunol. 2005; 137:66–72.

4. Zanconato S, Carraro S, Corradi M, Alinovi R, Pasquale MF, Piacentini G, et al. Leukotrienes and 8-isoprostane in exhaled breath condensate of children with stable and unstable asthma. J Allergy Clin Immunol. 2004; 113:257–263.

5. Csoma Z, Kharitonov SA, Balint B, Bush A, Wilson NM, Barnes PJ. Increased leukotrienes in exhaled breath condensate in childhood asthma. Am J Respir Crit Care Med. 2002; 166:1345–1349.

6. Papadopoulos NG, Arakawa H, Carlsen KH, Custovic A, Gern J, Lemanske R, et al. International consensus on (ICON) pediatric asthma. Allergy. 2012; 67:976–997.

7. Montuschi P, Barnes PJ. Exhaled leukotrienes and prostaglandins in asthma. J Allergy Clin Immunol. 2002; 109:615–620.

8. Kostikas K, Gaga M, Papatheodorou G, Karamanis T, Orphanidou D, Loukides S. Leukotriene B4 in exhaled breath condensate and sputum supernatant in patients with COPD and asthma. Chest. 2005; 127:1553–1559.

9. Krawiec ME, Westcott JY, Chu HW, Balzar S, Trudeau JB, Schwartz LB, et al. Persistent wheezing in very young children is associated with lower respiratory inflammation. Am J Respir Crit Care Med. 2001; 163:1338–1343.

10. Tomassini M, Magrini L, De Petrillo G, Adriani E, Bonini S, Balsano F, et al. Serum levels of eosinophil cationic protein in allergic diseases and natural allergen exposure. J Allergy Clin Immunol. 1996; 97:1350–1355.

11. Cianchetti S, Bacci E, Ruocco L, Pavia T, Bartoli ML, Cardini C, et al. Are sputum eosinophil cationic protein and eosinophils differently associated with clinical and functional findings of asthma? Clin Exp Allergy. 2014; 44:673–680.

12. Kim KW, Jee HM, Park YH, Choi BS, Sohn MH, Kim KE. Relationship between amphiregulin and airway inflammation in children with asthma and eosinophilic bronchitis. Chest. 2009; 136:805–810.

13. Montuschi P. Analysis of exhaled breath condensate in respiratory medicine: methodological aspects and potential clinical applications. Ther Adv Respir Dis. 2007; 1:5–23.

14. Scheideler L, Manke HG, Schwulera U, Inacker O, Hammerle H. Detection of nonvolatile macromolecules in breath. A possible diagnostic tool? Am Rev Respir Dis. 1993; 148:778–784.

15. Fujimoto K, Kubo K, Matsuzawa Y, Sekiguchi M. Eosinophil cationic protein levels in induced sputum correlate with the severity of bronchial asthma. Chest. 1997; 112:1241–1247.

16. Seggev JS, Wiessner JH, Thornton WH Jr, Edes TE. Comparison of serum and plasma leukotriene B4 levels in normal and asthmatic subjects. Ann Allergy Asthma Immunol. 1995; 75:365–368.
17. Gleich GJ, Adolphson CR. The eosinophilic leukocyte: structure and function. Adv Immunol. 1986; 39:177–253.

18. Venge P, Bystrom J, Carlson M, Hakansson L, Karawacjzyk M, Peterson C, et al. Eosinophil cationic protein (ECP): molecular and biological properties and the use of ECP as a marker of eosinophil activation in disease. Clin Exp Allergy. 1999; 29:1172–1186.

19. Koh GC, Shek LP, Goh DY, Van Bever H, Koh DS. Eosinophil cationic protein: is it useful in asthma? A systematic review. Respir Med. 2007; 101:696–705.

20. Wolthers OD. Eosinophil granule proteins in the assessment of airway inflammation in pediatric bronchial asthma. Pediatr Allergy Immunol. 2003; 14:248–254.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download