Abstract
The response to drug treatment in asthma is a complex trait and is markedly variable even in patients with apparently similar clinical features. Pharmacogenomics is a study of variations of human genome characteristics as related to drug response. A traditional candidate-gene approach and genome-wide association studies have provided an increasing list of genes and variants that was associated with asthma medications. However, as phenotypic variations arises from a network of complex interactions among genetic and environmental factors, rather than individual genes, a multidisciplinary, system-level approach is required in order to understand the interrelationships among these factors. Systems biology that studies organisms as integrated and interacting networks of genes, proteins and biochemical reactions can contribute to this. It is likely that the combination of network modeling, functional validation, and integrative-Omics will be needed to move asthma pharmacogenomics closer to clinical relevance.
Figures and Tables
Fig. 2
Components of system. Node, basic component (e.g. each gene, protein, or metabolite); line, relation between nodes; hub, important node.
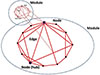
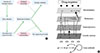
Fig. 3
Application of systems biology in pharmacogenomics. (A) outline, (B) Example. g, genetic variant; t, transcript; p, protein; m, metabolite.
References
1. Szefler SJ, Martin RJ, King TS, Boushey HA, Cherniack RM, Chinchilli VM, et al. Significant variability in response to inhaled corticosteroids for persistent asthma. J Allergy Clin Immunol. 2002; 109:410–418.

2. Malmstrom K, Rodriguez-Gomez G, Guerra J, Villaran C, Pineiro A, Wei LX, et al. Montelukast/Beclomethasone Study Group. Oral montelukast, inhaled beclomethasone, and placebo for chronic asthma. A randomized, controlled trial. Ann Intern Med. 1999; 130:487–495.

3. Drazen JM, Silverman EK, Lee TH. Heterogeneity of therapeutic responses in asthma. Br Med Bull. 2000; 56:1054–1070.

4. Szefler SJ, Phillips BR, Martinez FD, Chinchilli VM, Lemanske RF, Strunk RC, et al. Characterization of within-subject responses to fluticasone and montelukast in childhood asthma. J Allergy Clin Immunol. 2005; 115:233–242.

5. U.S. Department of Health and Human Services. Food and Drug Administration. Center for Drug Evaluation and Research (CDER). Center for Biologics Evaluation and Research (CBER). Guidance for industry: E15 definitions for genomic biomarkers, pharmacogenomics, pharmacogenetics, genomic data and sample coding categories. Silver Spring, MD: U.S. Food and Drug Administration;cited 2014 Nov 11. Available from: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm073162.pdf.
6. Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010; 363:809–819.

7. Chen P, Lin JJ, Lu CS, Ong CT, Hsieh PF, Yang CC, et al. Carbamazepine-induced toxic effects and HLA-B*1502 screening in Taiwan. N Engl J Med. 2011; 364:1126–1133.

8. Corren J, Lemanske RF, Hanania NA, Korenblat PE, Parsey MV, Arron JR, et al. Lebrikizumab treatment in adults with asthma. N Engl J Med. 2011; 365:1088–1098.

9. FDA approves updated warfarin (coumadin) prescribing information: new genetic information may help providers improve initial dosing estimates of the anticoagulant for individual patients. Silver Spring, MD: U.S. Food and Drug Administration;cited 2014 Nov 11. Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2007/ucm108967.htm.
10. Israel E, Chinchilli VM, Ford JG, Boushey HA, Cherniack R, Craig TJ, et al. Use of regularly scheduled albuterol treatment in asthma: genotype-stratified, randomised, placebo-controlled cross-over trial. Lancet. 2004; 364:1505–1512.

11. Giacomini KM, Brett CM, Altman RB, Benowitz NL, Dolan ME, Flockhart DA, et al. The pharmacogenetics research network: from SNP discovery to clinical drug response. Clin Pharmacol Ther. 2007; 81:328–345.

12. McCarthy JJ, Hilfiker R. The use of single-nucleotide polymorphism maps in pharmacogenomics. Nat Biotechnol. 2000; 18:505–508.

13. Evans WE, McLeod HL. Pharmacogenomics: drug disposition, drug targets, and side effects. N Engl J Med. 2003; 348:538–549.

14. Evans WE, Relling MV. Pharmacogenomics: translating functional genomics into rational therapeutics. Science. 1999; 286:487–491.

15. Evans WE, Relling MV. Moving towards individualized medicine with pharmacogenomics. Nature. 2004; 429:464–468.

16. Litonjua AA, Lasky-Su J, Schneiter K, Tantisira KG, Lazarus R, Klanderman B, et al. ARG1 is a novel bronchodilator response gene: screening and replication in four asthma cohorts. Am J Respir Crit Care Med. 2008; 178:688–694.
17. Duan QL, Du R, Lasky-Su J, Klanderman BJ, Partch AB, Peters SP, et al. A polymorphism in the thyroid hormone receptor gene is associated with bronchodilator response in asthmatics. Pharmacogenomics J. 2013; 13:130–136.

18. Tantisira KG, Lake S, Silverman ES, Palmer LJ, Lazarus R, Silverman EK, et al. Corticosteroid pharmacogenetics: association of sequence variants in CRHR1 with improved lung function in asthmatics treated with inhaled corticosteroids. Hum Mol Genet. 2004; 13:1353–1359.

19. Tantisira KG, Hwang ES, Raby BA, Silverman ES, Lake SL, Richter BG, et al. TBX21: a functional variant predicts improvement in asthma with the use of inhaled corticosteroids. Proc Natl Acad Sci U S A. 2004; 101:18099–18104.

20. Drazen JM, Yandava CN, Dube L, Szczerback N, Hippensteel R, Pillari A, et al. Pharmacogenetic association between ALOX5 promoter genotype and the response to anti-asthma treatment. Nat Genet. 1999; 22:168–170.

21. Klotsman M, York TP, Pillai SG, Vargas-Irwin C, Sharma SS, van den Oord EJ, et al. Pharmacogenetics of the 5-lipoxygenase biosynthetic pathway and variable clinical response to montelukast. Pharmacogenet Genomics. 2007; 17:189–196.

22. Himes BE, Jiang X, Hu R, Wu AC, Lasky-Su JA, Klanderman BJ, et al. Genome-wide association analysis in asthma subjects identifies SPATS2L as a novel bronchodilator response gene. PLoS Genet. 2012; 8:e1002824.

23. Drake KA, Torgerson DG, Gignoux CR, Galanter JM, Roth LA, Huntsman S, et al. A genome-wide association study of bronchodilator response in Latinos implicates rare variants. J Allergy Clin Immunol. 2014; 133:370–378.

24. Tantisira KG, Lasky-Su J, Harada M, Murphy A, Litonjua AA, Himes BE, et al. Genomewide association between GLCCI1 and response to glucocorticoid therapy in asthma. N Engl J Med. 2011; 365:1173–1183.

25. Tantisira KG, Damask A, Szefler SJ, Schuemann B, Markezich A, Su J, et al. Genome-wide association identifies the T gene as a novel asthma pharmacogenetic locus. Am J Respir Crit Care Med. 2012; 185:1286–1291.

26. Park HW, Dahlin A, Tse S, Duan QL, Schuemann B, Martinez FD, et al. Genetic predictors associated with improvement of asthma symptoms in response to inhaled corticosteroids. J Allergy Clin Immunol. 2014; 133:664–669.e5.

27. Maitland-van der Zee AH, Boerwinkle E. Pharmacogenetics of response to statins: where do we stand? Curr Atheroscler Rep. 2005; 7:204–208.

28. Wilke RA, Reif DM, Moore JH. Combinatorial pharmacogenetics. Nat Rev Drug Discov. 2005; 4:911–918.

29. Sing CF, Stengard JH, Kardia SL. Dynamic relationships between the genome and exposures to environments as causes of common human diseases. World Rev Nutr Diet. 2004; 93:77–91.

30. Moore JH. The ubiquitous nature of epistasis in determining susceptibility to common human diseases. Hum Hered. 2003; 56:73–82.

31. Thornton-Wells TA, Moore JH, Haines JL. Genetics, statistics and human disease: analytical retooling for complexity. Trends Genet. 2004; 20:640–647.

32. Quackenbush J. Extracting meaning from functional genomics experiments. Toxicol Appl Pharmacol. 2005; 207:2 Suppl. 195–199.

33. Gajendran VK, Lin JR, Fyhrie DP. An application of bioinformatics and text mining to the discovery of novel genes related to bone biology. Bone. 2007; 40:1378–1388.

34. Nenadic G, Spasic I, Ananiadou S. Terminology-driven mining of biomedical literature. Bioinformatics. 2003; 19:938–943.

35. Yandell MD, Majoros WH. Genomics and natural language processing. Nat Rev Genet. 2002; 3:601–610.

36. Davies SM. Pharmacogenetics, pharmacogenomics and personalized medicine: are we there yet? Hematology Am Soc Hematol Educ Program. 2006; 111–117.

37. Ritchie MD. Bioinformatics approaches for detecting gene-gene and gene-environment interactions in studies of human disease. Neurosurg Focus. 2005; 19:E2.

38. Moore JH, Ritchie MD. STUDENTJAMA. The challenges of whole-genome approaches to common diseases. JAMA. 2004; 291:1642–1643.
40. Koster ES, Rodin AS, Raaijmakers JA, Maitland-van der Zee AH. Systems biology in pharmacogenomic research: the way to personalized prescribing? Pharmacogenomics. 2009; 10:971–981.

42. Morel NM, Holland JM, van der Greef J, Marple EW, Clish C, Loscalzo J, et al. Primer on medical genomics. Part XIV: Introduction to systems biology: a new approach to understanding disease and treatment. Mayo Clin Proc. 2004; 79:651–658.

43. Dahlin A, Tantisira KG. Integrative systems biology approaches in asthma pharmacogenomics. Pharmacogenomics. 2012; 13:1387–1404.

44. Needham CJ, Bradford JR, Bulpitt AJ, Westhead DR. A primer on learning in Bayesian networks for computational biology. PLoS Comput Biol. 2007; 3:e129.

45. Friedman N, Linial M, Nachman I, Pe'er D. Using Bayesian networks to analyze expression data. J Comput Biol. 2000; 7:601–620.

46. Beerenwinkel N, Siebourg J. Probability, statistics, and computational science. Methods Mol Biol. 2012; 855:77–110.

47. Himes BE, Wu AC, Duan QL, Klanderman B, Litonjua AA, Tantisira K, et al. Predicting response to short-acting bronchodilator medication using Bayesian networks. Pharmacogenomics. 2009; 10:1393–1412.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download