Abstract
Purpose
Bronchial hyperresponsiveness is considered as a hallmark of asthma. The duration of asthma was demonstrated to be associated with bronchial responsiveness, expressed as methacholine PC20. We investigated the relationship between duration of asthma and percentage fall in forced vital capacity (FVC) at PC20 (ΔFVC), another index of bronchial responsiveness, which reflects excessive bronchoconstriction.
Methods
Six- to 8-year-old children with asthma underwent methacholine inhalation test. The PC20 and ΔFVC were calculated for each individual. The subjects were classified into those with wheezing onset in the first three years of life (early-onset asthma [EA], n=63) and those with wheezing onset from three years onwards (late-onset asthma [LA], n=99).
Results
From the time of wheezing onset, duration of asthma ranged from 0.2 to 8.3 years. The mean duration of asthma in patients with EA was 5.6 years (standard deviation [SD], 1.2 years), compared with 2.2 years (SD, 1.3 years) in the patients with LA. Patients with EA had a significantly lower forced expiratory volume in 1 second/FVC than did those with LA (84.6%±5.9% vs. 86.8%±5.1%, P<0.05). The ΔFVC was significantly higher in patients with EA than in those with LA (19.4%±5.1% vs. 17.0%±4.5%, P<0.01), but PC20 was not different between the two groups. In total subjects, asthma duration correlated significantly with ΔFVC (r=0.222, P<0.01), but not with PC20.
Bronchial hyperresponsiveness (BHR) is a characteristic feature of asthma, and is considered to be one of the major consequences of airway inflammation and remodeling.1) BHR is usually defined as an increased sensitivity of the airways to inhaled nonsensitizing bronchoconstrictor stimuli,2) which is evaluated by measuring the dose or concentration (PC20) of inhaled methacholine or histamine that causes a 20% fall in forced expiratory volume in 1 second (FEV1) from baseline.3) However, there is accumulating evidence that BHR is a more complex functional abnormality that comprises more than just hypersensitivity.4) Recently, Gibbons et al.5) proposed a novel indirect method for detection of excessive bronchoconstriction in patients with mild, newly diagnosed asthma. They retrospectively measured the percentage fall in forced vital capacity (FVC) at PC20 (ΔFVC), which reflects gas trapped at that point of the dose-response curve due to excessive bronchoconstriction, and found that this index of airway closure was not related to the PC20.
A number of cross-sectional studies have shown that duration of asthma was associated with lower levels of lung function.6,7,8,9,10) Moreover, multiyear prospective studies of both children11,12) and adults13,14) with asthma revealed worsening pulmonary function over time, which suggest that childhood asthma seems to be associated with reduced lung function growth, and adult asthmatics may also have increased decline in lung function during their life. However, a few studies have tried to relate the duration of asthma to bronchial responsiveness in the patients with asthma.6,15,16) The Childhood Asthma Management Program (CAMP) study demonstrated that duration of disease correlated negatively with levels of bronchial responsiveness in children with mild-to-moderate asthma.6) Such a relationship was also founded in adolescents with outgrown childhood asthma,15) and adults with asthma.16) These studies suggest that disease progression may be associated with worsening of bronchial responsiveness, which has been assessed by measuring PC20. However, little is known about the relationship between the duration of asthma and another index of bronchial responsiveness, ΔFVC.
The aims of the present study were to evaluate whether duration of disease is associated with ΔFVC as well as PC20, and to examine the relationship between the levels of lung function and duration of disease in children with asthma.
One hundred sixty-two children with asthma, aged 6-8 years were recruited from the allergy clinic at Seoul National University Children's Hospital. All subjects had a history of more than three episodes of wheezing, and mild to severe asthmatic symptoms, which are controlled by inhaled bronchodilators on an as-needed basis, with or without the regular use prophylactic medications. To be eligible for the study, all patients (1) had a history of at least one episode of wheezing in the last 12 months, (2) had a FEV1 more than 70% of the predicted value, and (3) were required to have a methacholine PC20 below 16 mg/mL.
To examine the association between duration of asthma and clinical parameters including lung function and bronchial responsiveness, the subjects were grouped according to the onset of wheezing: early-onset asthma (EA, children with wheezing onset in the first 3 years of life; n=63), late-onset asthma (LA, children with wheezing onset from 3 years onwards; n=99). Asthma duration was calculated by subtracting the age of the participants at the time of methacholine inhalation test from the age of the subjects at the time of wheezing onset. Data were collected by questionnaires regarding history of asthma symptoms, current symptom frequency (over the last month), and treatment of asthma, and were confirmed by chart review. Asthma severity on a scale of mild intermittent, mild persistent, moderate persistent, severe persistent was based on symptom frequency and medication use suggested by the National Asthma Education and Prevention Program guidelines.17)
Skin prick tests were performed on all children to evaluate atopic status. All subjects provided blood samples for the determination of total eosinophil counts and serum total IgE levels, and all underwent methacholine challenge tests. The patients stopped using inhaled bronchodilators or other medications 48 hours and inhaled corticosteroids 7 days before the study days. There was no history of upper respiratory infections for at least 4 weeks before the study.
Methacholine inhalation tests were carried out using a modification of the method described by Chai et al.18) Spirometric measurements (FEV1 and FVC) were made using a computerized spirometer (Microspiro-HI 298, Chest, Tokyo, Japan), in accordance with the recommendations of the American Thoracic Society (ATS).19) The time course of the preceding inspiration was standardized, i.e., rapid maximal inspiration without an end-inspiratory pause, and the FVC maneuver was continued until a pause in the forced expired volume curve was obvious by visual inspection; the minimum duration of the FVC maneuver was 6 seconds. Subjects who were unable to perform spirometric tests reproducibly or who had a low FEV1 (<70% predicted)20) were excluded. Methacholine (Sigma Diagnostics, St. Louis, MO, USA) solutions were prepared at different concentrations (0.075, 0.15, 0.3, 0.625, 1.25, 2.5, 5, 10, and 25 mg/mL) in buffered saline solution (pH 7.4). A Rosenthal-French dosimeter (Laboratory for Applied Immunology, Baltimore, MD, USA), triggered by a solenoid valve set to remain open for 0.6 second, was used to generate the aerosol from a DeVilbiss 646 nebulizer (DeVilbiss Health Care, Somerset, PA, USA), with pressurized air at 20 pounds per square inch. Each subject inhaled five inspiratory capacity breaths of buffered saline solution and increasing concentrations of methacholine at 5-minute intervals. This gave an output of 0.009±0.0014 mL (mean±standard deviation [SD]) per inhalation. FEV1 and FVC were measured 60 to 90 seconds after inhalation at each concentration level, and the largest value of triplicate FEV1 or FVC was used for analysis. The procedure was terminated when the FEV1 level fell by >20% of the postsaline solution value, or the highest concentration was reached. The methacholine PC20 was obtained from the log concentration-percent fall in FEV1 curve by linear interpolation of the last two points. The ΔFVC relative to baseline FVC after saline inhalation was also calculated using log-linear interpolation.
Atopy was defined as at least one positive skin-prick test response (wheal size>3 mm) in a panel of 12 common aeroallergens, in the presence of positive and negative controls. The majority of subjects showed a positive skin reaction to house dust mites.
Total serum IgE was measured using the Coat-A-Count Total IgE IRMA (Diagnostic Products Co., Loa Angeles, CA, USA). Whole blood was collected by sterile venipuncture, serum was separated, and stored frozen (-80℃) until assayed. Determinations were made according to manufacturer's specifications.
Blood samples were withdrawn using a 21-gauge butterfly needle with an attached syringe; care was taken to avoid hemolysis. Eosinophil numbers were counted using an automated hematology analyzer (Coulter Counter, STKS, Beckman Coulter, Fullerton, CA, USA).
The study was approved by the Hospital Ethics Committee, and the parents of all children gave their informed consent.
The values of FEV1 and FVC are expressed as percentages of predicted based on data from our local population.20) The values for PC20, blood total eosinophil count, and serum total IgE were logarithmically transformed before analysis and are expressed as geometric means with a range of 1 SD. Other values are presented as mean±1 SD. Values of subjects with EA and LA were compared using the Student t-test. Pearson correlation test or nonparametric Spearman rank method was used to analyze relations between variables. Standardized β coefficients were used for multiple regression models to allow comparison of the relative impact of the variables. A P-value of less than 0.05 was considered statistically significant.
Demographic characteristics are summarized in Table 1. Subjects ranged in age from 6 to 8 years with a mean age of 7.6 years (median, 7.4 years). There was no significant difference in age between the groups with EA and LA. The majority of the subjects was male (72.8%); no difference between the two groups (EA, 74.6%; LA, 71.7%). The duration of asthma in total subjects was a mean of 3.5 years (SD, 2.1 years; median, 3.5 years; range, 0.2 to 8.3 years). The mean duration of asthma in the patients with EA was 5.6 years (SD, 1.2 years; median, 5.5 years; range, 3.6 to 8.3 years), compared with 2.2 years in the patients with LA (SD, 1.4 years; median, 2.2 years; range, 0.2 to 5.4 years). The age of wheezing onset varied among study subjects, ranged from 0.1 to 8.8 years with mean age of 4.1 years (SD, 2.2 years; median, 3.8 years). The age of wheezing onset in the patients with EA was a mean of 1.9 years (SD, 0.9 years; median, 2.1 years; range, 0.1 to 3.0 years), compared with a mean of 5.4 years in the patients with LA (SD, 1.5 years; median, 5.5 years; range, 3.0 to 8.8 years). The value for height was not significantly different between the two groups.
As shown in Table 2, the majority of total subjects (90.1%), as well as the majority of patients with EA and LA (88.9% and 90.9%, respectively), had mild to moderate asthma as determined by symptom frequency and medication use. Although the proportion of moderate to severe asthma was slightly higher in the patients with EA (44.4%) than in those with LA (38.4%), the difference was not statistically significant. The prevalence of inhaled corticosteroid usage and oral corticosteroid in the previous 6 months was 58.7%, 20.6%, respectively in the patient with EA, not significantly different from 55.6%, 17.2%, respectively in the patients with LA. The patients with EA and those with LA did not differ in terms of atopy, as evidenced by total serum IgE measurement and skin prick test. The levels of total serum IgE was not different between the patients with EA and LA (EA [geometric mean, 332.8 IU/mL; range of 1SD, 76.5 to 1,447.3], LA [geometric mean, 311.2 IU/mL; range of 1SD, 84.6 to 1,144.4], respectively). Likewise, there was no significant difference in the percentage of patients with EA and with LA who had a positive response to at least one of the allergens tested (84.1%, 78.8%, respectively). The geometric mean value of blood total eosinophil count was not significantly different between the two groups (EA: 422.2/µL, 221.7 to 804.0; LA: 400.0/µL, 191.4 to 836.0, respectively).
Baseline FEV1 was 1.38±0.22 L in the patients with EA and 1.43±0.20 L in those with LA, and was not significantly different between the two groups. There was a trend toward a lower FEV1 in the EA (93.1%±10.6%) than in the LA group (96.0%±10.6%), when FEV1 was expressed as a percentage of predicted values (P=0.095). Baseline FVC in the patients with EA (1.64±0.26 L) was not significantly different from that in the patients with LA (1.66±0.25 L). Patients with EA had a significantly lower FEV1/FVC than did those with LA (84.6%±5.9%, 86.8%±5.1%, respectively, P<0.05). The ΔFVC was significantly higher in patients with EA than in those with LA (19.4%±5.1%, 17.0%±4.5%, respectively, P<0.01), but PC20 was not different between the two groups (EA: 1.75 mg/mL, 0.61 to 4.99; LA: 1.92 mg/mL, 0.46 to 8.08) (Table 3).
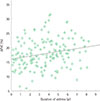
An inverse correlation was observed between the duration of asthma and FEV1/FVC (r=-0.162, P<0.05), but there was no significant correlation between asthma duration and FEV1 percent predicted (r=-0.107, P=0.175). Asthma duration correlated significantly with ΔFVC (r=0.222, P<0.01) (Fig. 1), but not with PC20 (r=-0.027, P=0.730). All other clinical parameters including age, serum total IgE, and blood total eosinophil count did not show statistically significant correlations with duration of asthma (r=0.094 [P=0.233], r=0.035 [P=0.656], r=0.066 [P=0.404], respectively). The FEV1/FVC ratio also correlated with asthma severity (r=-0.372, P<0.001). The correlation between the duration of asthma and FEV1/FVC was not statistically significant, when adjusted for asthma severity (β=-0.127, P=0.08).
ΔFVC correlated significantly with FEV1/FVC (r=-0.406, P<0.001) as well as duration of asthma. ΔFVC also showed a significantly negative correlation with asthma severity (r=0.282, P<0.001). Neither PC20 nor FEV1 percent predicted correlated with ΔFVC (r=-0.067 [P=0.399], r=-0.103 [P=0.191], respectively). In addition, ΔFVC did not correlate with all other demographic and clinical continuous variables (data not shown). Multiple linear regression models were used to determine the relationship between ΔFVC and several parameters of asthma duration and severity, and FEV1/FVC. In Table 4, ΔFVC was significantly associated with duration of disease (P<0.05), independent of asthma severity and FEV1/FVC.
This study showed lower levels of FEV1/FVC and higher levels of ΔFVC in subjects with EA compared to those with LA. But the levels of PC20 were not different between the two groups. Likewise, asthma duration was found to be associated with lower levels of FEV1/FVC, higher levels of ΔFVC, but not with levels of PC20 in total subjects.
CAMP study defined duration of asthma from time of diagnosis.6) However, the interval from the time of wheezing onset to diagnosis may vary between individuals and the standards of asthma diagnosis have considerably changed over years. Therefore, duration of asthma in the present study was defined from the time of onset of wheezing possibly suggestive of asthma rather than time of physician diagnosis. Our subjects were six years of age or over at which a child develops the ability to perform forced expiratory maneuvers reliably, thus recruitment of children with this age allows us to detect changes in lung function and bronchial responsiveness by the duration of asthma, using this maneuver as early as possible in childhood. To enroll more subjects, we selected children who ranged from six to eight years of age, and classified them into the two groups by onset of wheezing. The cutoff value of 3 years of age as used to define groups by wheezing onset was derived from the classification of children with wheezing as described by the Tucson study.21) This classification of the subjects with a range of 6 to 8 years of age might be not ideal to study the relation of duration of disease to the other parameters, because age could affect the duration of disease. But duration of disease was significantly different between the two groups, while age was not different. Furthermore we also performed correlation analyses on data from the total study subjects.
Zeiger et al.6) found that asthma duration was associated with lower levels of FEV1 percent predicted and FEV1/FVC. This result was somewhat different from our finding that asthma duration correlated with FEV1/FVC negatively, but did not with FEV1 percent predicted. Our study population was too small to detect the modest level of correlation observed in the CAMP data.6) This problem is particularly acute in most clinical investigations of asthma study participants, because there is a tendency to truncate the range of pulmonary function values in the clinical population under investigation, thus potentially reducing the opportunity to observe correlations. Furthermore, an increase in the asthma duration range might be expected to demonstrate a weak association of lung function, expressed as FEV1 with asthma duration. However, the association between FEV1/FVC and asthma duration in the present study was not significant, when adjusted for asthma severity, which is in agreement with previous study.22) Asthma severity seems to be related better to decreased pulmonary function than duration of asthma.
The levels of PC20, an index of bronchial responsiveness were found to be not different between the two asthma groups, and did not correlate with duration of asthma in total subjects. It reflects the value of PC20 might not be affected by duration of asthma, although it is possible that our results were different from previous findings that showed the relationship between the two parameters,6,23) probably because of small sample size and/or relatively short range of asthma duration as mentioned above. In contrast, the levels of ΔFVC were different between EA and LA groups, and were associated with duration of asthma in total subjects. Moreover, it is important that despite the small size of our study population, we were able to identify a significant association between the two parameters. ΔFVC has been suggested to be a useful index of disease severity in asthma.24,25) Therefore, it should be mentioned that the EA group did not differ statistically from the other group, in terms of severity of disease. Furthermore, the ΔFVC remained significantly associated with duration of asthma for whole study group, when adjusted for severity of disease. Recently, inhaled corticosteroids were reported to have an effect on the levels of ΔFVC.26) Although inhaled corticosteroids were discontinued at least one week before the study, they may have carry-over effects. However, the prevalence of current inhaled and oral steroid usage was not significantly different between the two groups, though neither the corticosteroid dose nor duration of this therapy could be confirmed. Therefore it seems justified to assume that severity of disease and the previous steroid treatment did not bias our results. We also found the association between FEV1/FVC and ΔFVC. This raised questions about the effects of the duration of asthma on the degree of ΔFVC. However, our multiple regression model for ΔFVC suggests increased ΔFVC was associated with duration of disease independent of airflow limitation at baseline.
It should be mentioned that mean ΔFVC in the present study is higher than those previously reported in adult asthmatics.5,24,25,26) This is unlikely to be due to difference in the disease severity, because our levels were higher than those of even severe adult asthmatics in previous study.25) Children may be prone to muscle weakness and fatigue, and it is possible that the observed increase in ΔFVC may be a consequence of reduction in FVC during bronchoprovocation test, which is caused by progressive shortening in expiration leading to incomplete emptying of the lungs. However, this is not the case, since the compliance with ATS criteria,19) including the occurrence of an expiratory plateau, was checked in all cases. The loss of elastic support to the airways was suggested to be responsible for the higher level of ΔFVC in elderly subjects with asthma.27) A detailed study of pulmonary elasticity during the period of growth showed that it increases with age and reaches a maximum in the late teens.28) We speculate that age-related factors in lung elasticity may result in an enhanced bronchoconstriction, and thus higher ΔFVC.
In the present study, prolonged asthma was associated with higher levels of ΔFVC, but not lower levels of PC20. In addition, there was no correlation between PC20 and ΔFVC, which was also the case in the patients described by other investigators.5,25,29) The mechanisms of hypersensitivity include epithelial malfunction or inflammatory cell recruitment and activity,4) whereas the mechanisms of excessive airway narrowing include inflammatory structural changes such as increased airway muscle4,30,31) or exudative airway wall thickness,4,32) which could be partially called remodeling.33) Computational analyses of postmortem lungs by Pare, Hogg, and colleagues31,34,35) have indicated that a modest degree of airway wall thickening, can exaggerate the airway closure induced by airway smooth muscle shortening. There are some epidemiologic evidences for relationship between duration of asthma and remodeling.7,9) Cassino et al.7) demonstrated that duration of asthma was inversely associated with FEV1 percent predicted and most patients with asthma of long duration failed to achieve normal airflow after bronchodilator administration, indicating that decrement in pulmonary function associated with long-term asthma may become irreversible. In addition, Rasmussen et al.36) suggest that airway remodeling in asthma, which was evaluated using postbronchodilator FEV1/FVC ratio as a surrogate, begins in childhood and continues into adult life. Moreover, they demonstrated that airway remodeling was associated with an EA. The hypothesis that if airway remodeling is related to duration of asthma, then the airways of asthmatics with a longer duration of disease should show greater alterations is also supported by radiological37) and histopathological evidences.38) Therefore these results could explain why duration of asthma was associated with ΔFVC but not with PC20. Although underemphasized, the effects of lung elastic recoil and peribronchial inflammation on airway smooth muscle load also important roles in determining the degree of bronchoconstriction.39,40) Recently, duration of asthma was demonstrated to be associated with degree of hyperinflation, which might result from a loss of elastic recoil as well as airflow obstruction.7) These observations suggest an additional mechanism for relationship of duration of asthma to ΔFVC.
In conclusion, the inverse correlation of asthma duration with bronchial responsiveness noted in the present study suggests that prolonged asthma is associated with worsened bronchial responsiveness during childhood, and such an association with bronchial responsiveness may be reflected by ΔFVC. Longitudinal studies are needed in large numbers to clarify the significance of these relationships.
Figures and Tables
Fig. 1
Correlation between duration of asthma and ΔFVC (r=0.222, P<0.05). ΔFVC, forced vital capacity at PC20.
References
1. Brannan JD, Lougheed MD. Airway hyperresponsiveness in asthma: mechanisms, clinical significance, and treatment. Front Physiol. 2012; 3:460.

2. Hargreave FE, Dolovich J, O'Byrne PM, Ramsdale EH, Daniel EE. The origin of airway hyperresponsiveness. J Allergy Clin Immunol. 1986; 78(5 Pt 1):825–832.

3. Cockcroft DW, Killian DN, Mellon JJ, Hargreave FE. Bronchial reactivity to inhaled histamine: a method and clinical survey. Clin Allergy. 1977; 7:235–243.

4. Sterk PJ, Bel EH. Bronchial hyperresponsiveness: the need for a distinction between hypersensitivity and excessive airway narrowing. Eur Respir J. 1989; 2:267–274.
5. Gibbons WJ, Sharma A, Lougheed D, Macklem PT. Detection of excessive bronchoconstriction in asthma. Am J Respir Crit Care Med. 1996; 153:582–589.

6. Zeiger RS, Dawson C, Weiss S. Relationships between duration of asthma and asthma severity among children in the Childhood Asthma Management Program (CAMP). J Allergy Clin Immunol. 1999; 103(3 Pt 1):376–387.

7. Cassino C, Berger KI, Goldring RM, Norman RG, Kammerman S, Ciotoli C, et al. Duration of asthma and physiologic outcomes in elderly nonsmokers. Am J Respir Crit Care Med. 2000; 162(4 Pt 1):1423–1428.

8. Hudon C, Turcotte H, Laviolette M, Carrier G, Boulet LP. Characteristics of bronchial asthma with incomplete reversibility of airflow obstruction. Ann Allergy Asthma Immunol. 1997; 78:195–202.

9. Little SA, MacLeod KJ, Chalmers GW, Love JG, McSharry C, Thomson NC. Association of forced expiratory volume with disease duration and sputum neutrophils in chronic asthma. Am J Med. 2002; 112:446–452.

10. Olaguibel Rivera JM, Alvarez-Puebla MJ, Puy Uribe San Martín M, Tallens Armand ML. Duration of asthma and lung function in life-long nonsmoking adults. J Investig Allergol Clin Immunol. 2007; 17:236–241.
11. Agertoft L, Pedersen S. Effects of long-term treatment with an inhaled corticosteroid on growth and pulmonary function in asthmatic children. Respir Med. 1994; 88:373–381.

12. Porpodis K, Papakosta D, Manika K, Kontakiotis T, Gaga M, Sichletidis L, et al. Long-term prognosis of asthma is good--a 12-year follow-up study. Influence of treatment. J Asthma. 2009; 46:625–631.

13. Dompeling E, van Schayck CP, van Grunsven PM, van Herwaarden CL, Akkermans R, Molema J, et al. Slowing the deterioration of asthma and chronic obstructive pulmonary disease observed during bronchodilator therapy by adding inhaled corticosteroids. A 4-year prospective study. Ann Intern Med. 1993; 118:770–778.

14. Limb SL, Brown KC, Wood RA, Wise RA, Eggleston PA, Tonascia J, et al. Irreversible lung function deficits in young adults with a history of childhood asthma. J Allergy Clin Immunol. 2005; 116:1213–1219.

15. Obase Y, Shimoda T, Kawano T, Saeki S, Tomari S, Izaki K, et al. Bronchial hyperresponsiveness and airway inflammation in adolescents with asymptomatic childhood asthma. Allergy. 2003; 58:213–220.

16. Boulet LP, Turcotte H, Laviolette M, Naud F, Bernier MC, Martel S, et al. Airway hyperresponsiveness, inflammation, and subepithelial collagen deposition in recently diagnosed versus long-standing mild asthma. Influence of inhaled corticosteroids. Am J Respir Crit Care Med. 2000; 162(4 Pt 1):1308–1313.

17. Urbano FL. Review of the NAEPP 2007 Expert Panel Report (EPR-3) on Asthma Diagnosis and Treatment Guidelines. J Manag Care Pharm. 2008; 14:41–49.

18. Chai H, Farr RS, Froehlich LA, Mathison DA, McLean JA, Rosenthal RR, et al. Standardization of bronchial inhalation challenge procedures. J Allergy Clin Immunol. 1975; 56:323–327.

19. American Thoracic Society. Standardization of Spirometry, 1994 Update. Am J Respir Crit Care Med. 1995; 152:1107–1136.
20. Yoon KA, Lim HS, Koh YY, Kim H. Normal predicted values of pulmonary function test in Korean school-aged children. J Korean Pediatr Soc. 1993; 36:25–37.
21. Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. The Group Health Medical Associates. Asthma and wheezing in the first six years of life. N Engl J Med. 1995; 332:133–138.

22. Kupczyk M, Kuprys I, Gorski P, Kuna P. Long-term deterioration of lung function in asthmatic outpatients. Respiration. 2004; 71:233–240.

23. Weiss ST, Van Natta ML, Zeiger RS. Relationship between increased airway responsiveness and asthma severity in the childhood asthma management program. Am J Respir Crit Care Med. 2000; 162:50–56.

24. Lee P, Abisheganaden J, Chee CB, Wang YT. A new asthma severity index: a predictor of near-fatal asthma? Eur Respir J. 2001; 18:272–278.

25. Abisheganaden J, Chan CC, Chee CB, Wang YT. Methacholine-induced fall in forced vital capacity as a marker of asthma severity. Respir Med. 1999; 93:277–282.

26. Oga T, Nishimura K, Tsukino M, Hajiro T, Ikeda A. Changes in indices of airway hyperresponsiveness during one year of treatment with inhaled corticosteroids in patients with asthma. J Asthma. 2001; 38:133–139.

27. Cuttitta G, Cibella F, Bellia V, Grassi V, Cossi S, Bucchieri S, et al. Changes in FVC during methacholine-induced bronchoconstriction in elderly patients with asthma: bronchial hyperresponsiveness and aging. Chest. 2001; 119:1685–1690.

28. Zapletal A, Paul T, Samanek M. Pulmonary elasticity in children and adolescents. J Appl Physiol. 1976; 40:953–961.

29. Lim TK, Ang SM. Excessive bronchoconstriction induced by histamine and effects of volume history in patients with bronchial asthma. Respirology. 1997; 2:107–112.

30. Lambert RK, Wiggs BR, Kuwano K, Hogg JC, Pare PD. Functional significance of increased airway smooth muscle in asthma and COPD. J Appl Physiol (1985). 1993; 74:2771–2781.

31. James AL, Pare PD, Hogg JC. The mechanics of airway narrowing in asthma. Am Rev Respir Dis. 1989; 139:242–246.

32. Kimura K, Inoue H, Ichinose M, Miura M, Katsumata U, Takahashi T, et al. Bradykinin causes airway hyperresponsiveness and enhances maximal airway narrowing. Role of microvascular leakage and airway edema. Am Rev Respir Dis. 1992; 146(5 Pt 1):1301–1305.

33. Busse W, Elias J, Sheppard D, Banks-Schlegel S. Airway remodeling and repair. Am J Respir Crit Care Med. 1999; 160:1035–1042.

34. Wiggs BR, Moreno R, Hogg JC, Hilliam C, Pare PD. A model of the mechanics of airway narrowing. J Appl Physiol (1985). 1990; 69:849–860.

35. Paré PD, Roberts CR, Bai TR, Wiggs BJ. The functional consequences of airway remodeling in asthma. Monaldi Arch Chest Dis. 1997; 52:589–596.
36. Rasmussen F, Taylor DR, Flannery EM, Cowan JO, Greene JM, Herbison GP, et al. Risk factors for airway remodeling in asthma manifested by a low postbronchodilator FEV1/vital capacity ratio: a longitudinal population study from childhood to adulthood. Am J Respir Crit Care Med. 2002; 165:1480–1488.

37. Niimi A, Matsumoto H, Amitani R, Nakano Y, Mishima M, Minakuchi M, et al. Airway wall thickness in asthma assessed by computed tomography. Relation to clinical indices. Am J Respir Crit Care Med. 2000; 162(4 Pt 1):1518–1523.

38. Bai TR, Cooper J, Koelmeyer T, Pare PD, Weir TD. The effect of age and duration of disease on airway structure in fatal asthma. Am J Respir Crit Care Med. 2000; 162(2 Pt 1):663–669.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download