Abstract
Purpose
Allergic rhinitis (AR) is regarded as a risk factor for asthma and bronchial hyperresponsiveness (BHR) is frequently observed in patients with AR. The purpose of this study is to analyze the characteristics of AR patients with BHR and identify factors that contribute to the incidence of BHR.
Methods
The medical records of a total of 176 children with AR were analyzed retrospectively. All patients were evaluated by performing spirometry and a methacholine challenge test.
Results
One hundred and fifty-five patients (88%) were classified as the BHR-negative group and 21 patients (12%) were classified as the BHR-positive group. Forced expiratory flow between 25% and 75% of vital capacity (FEF25-75 %predicted) was reduced, and total eosinphil counts, total immunoglobulin E (IgE) level, and serum specific IgE levels of Dermatophagoides pteronyssinus and Dermatophagoides farinae were higher in the BHR-positive group compared to the BHR-negative group. However, FEF25-75 was the only statistically significant predictor for the presence of BHR on multivariate logistic regression analysis. The cutoff value to distinguish BHR-positive subjects obtained from a receiver operating characteristics curve of FEF25-75 was 88.4%. A higher frequency of BHR was found in the group with a FEF25-75 less than 88.4%, and the sensitivity, specificity, positive predictive value and negative predictive value were 57.1%, 80.6%, 28.6%, and 93.3%, respectively.
Allergic rhinitis (AR) is characterized by typical symptoms induced by nasal immunoglobulin E (IgE)-mediated inflammatory response after allergen exposure.1) Symptoms of AR can affect quality of life more than symptoms of asthma do,2) and the prevalence in children reaches 23.4% to 31.2%.3,4) Asthma and AR are known to be closely related,5) and AR is regarded as a risk factor for the incidence of asthma.6) Since AR often precedes or is associated with asthma, the World Health Organization document, "Allergic Rhinitis and its Impact on Asthma (ARIA),"7) emphasized the role of AR as a risk factor in the development of asthma and recommended exploration of bronchial involvement in patients with AR.
As abnormalities of spirometric parameters are exhibited by AR patients without asthma, impaired values of forced expiratory volume in 1 second (FEV1) can be detected,3) and airway reversibility evidenced by increased FEV1 after a bronchodilator test has been reported.8) AR patients are also reported to have reduced forced expiratory flow between 25% and 75% of vital capacity (FEF25-75) as an early marker of bronchial damage.9) These findings are suggestive of bronchial involvement and damage in patients with AR without obvious symptoms of asthma. In addition, nasal lavage of asthmatic children showed Th2 polarization, which confirmed there is a pathophysiologic link between the upper and lower airway.10) It also has been reported that there is an association between nasal allergic inflammation maintained by eosinophil infiltration and bronchial obstruction indicated by an reduced FEV1.11)
Bronchial hyperresponsiveness (BHR) to natural stimuli such as exercise or chemical stimuli such as histamine or methacholine is a characteristic feature of asthma,12) and it is associated with an inflammatory response in the lower respiratory tract in patients with asthma.13) BHR is observed in more than one third of AR patients,14-16) and children with AR are more likely to have BHR compared to children without AR.4) BHR to methacholine among rhinitics is associated with subclinical asthma,17) and AR patients with BHR are at significantly higher risk of developing asthma compared to patients with normal bronchial provocation test results.18) Factors that can affect the development of BHR in patients with AR include nasal inflammation,19) the duration of AR,20) positive skin prick test (SPT) reactions to a larger number of allergens,14) total IgE levels,21) sputum eosinophil counts,22) perennial type of rhinitis,15) low FEF25-75,23) low FEF25-75/forced vital capacity (FVC) ratio,24) positive bronchodilation test results,25) a family history of asthma15) and a family history of BHR.26)
There has been a lot of researches on the predictors of BHR in patients with AR but this is the first time in Korean pediatric patients with short duration of AR and relatively good lung function compared to patients with asthma. The purpose of this study is to analyze the characteristics of AR patients with BHR who are thought to be at a higher risk of developing asthma compared with those without BHR and to identify factors that contribute to the incidence of BHR.
The Severance Hospital Institutional Review Board approved this study, and informed consent was obtained from the parents of the children. The medical records of 176 children between the ages of 5 and 16 years who visited Severance Children's Hospital Pediatric Allergy Clinic and diagnosed with AR were analyzed retrospectively. The diagnosis of AR was made based on a history of symptoms including rhinorrhea, sneezing, nasal congestion and itching apart from colds and positive SPT response results or the presence of allergen specific serum IgE (sIgE) to one or more aeroallergens.9) Exclusion criteria included asthma symptoms such as cough, wheezing, dyspnea and shortness of breath, asthma previously diagnosed by a physician, acute or chronic respiratory infections, and the use of nasal or inhaled corticosteroids during the previous four weeks.
We divided them into patients with BHR and those without BHR and compared the two groups. In addition, peripheral blood total eosinophil counts (TEC), total serum IgE levels and sIgE tests were measured. TEC were measured with a Sysmex XE-2100 automated blood cell analyzer (Sysmex, Kobe, Japan) and total serum IgE and sIgE levels were determined by the CAP radioallergosorbent technique (UniCAP, Pharmacia, Uppsala, Sweden).
sIgE tests for the six most common allergens in Korea (Dermatophagoides pteronyssinus, Dermatophagoides farinae, egg whites, cow's milk, German cockroach and Alternaria) were performed in 168 children. Atopy was defined as total IgE levels greater than 150 IU/mL or sIgE levels for one or more allergens greater than 0.35 KU/L. In 108 children seven years of age or older, SPT were performed using 12 common aeroallergens: house dust mites (D. pteronyssinus, D. farinae), animal dander (cat epithelia, dog epithelia), pollen (mugwort, rye grass, ragweed, alder, oak), mold (Aspergillus fumigatus, Alternaria alternata), and cockroach (Blatella germanica). After dropping the allergen solutions on the children's backs, a skin prick was performed using 26 G needles. Histamine and isotonic solution were used as positive and negative controls, respectively, and an average wheel diameter greater than 3 mm measured 15 minutes after performing the test was defined as positive. A positive SPT response for one or more allergens was also defined as atopy.
Spirometry was performed, short-acting bronchodilator responses were measured to evaluate bronchial reversibility, and MCTs were performed to evaluate BHR in all patients.
Spirometry was performed using a Jaeger MasterScreen IOS (Jaeger, Wurzburg, Germany). FEV1, FVC, FEV1/FVC, and FEF25-75 were measured before and after bronchodilator inhalation according to the American Thoracic Society guidelines.27) Maximum values among the three FVC maneuvers were recorded. A bronchodilation test was performed using 400 µg of salbutamol. ΔFEV1 was defined as the value of change in percent predicted FEV1 after salbutamol inhalation multiplied by 100 divided by the value of baseline percent predicted FEV1. Likewise, ΔFEF25-75 was defined as the value of change in percent predicted FEF25-75 after salbutamol inhalation multiplied by 100 divided by the value of baseline percent predicted FEV1. The reference values of spirometric parameters were based on the previous paper.28)
MCT was performed according to standardized procedures. Methacholine (Sigma Chemical, St Louis, MO, USA) was dissolved in a buffered saline solution at several concentrations (0.075, 0.15, 0.31, 0.62, 1.25, 2.5, 5, 10, 25, 50 mg/mL) and inhaled five times using a dosimeter with an increase in concentration at five-minute intervals. The concentration was increased until the FEV1 decreased more than 20% compared to that after buffered saline inhalation or the maximum cumulative dose had been administered. The provocative concentration causing a 20% decrease in FEV1 (PC20) was calculated using a dose-response curve. Subjects were considered to have BHR to methacholine if their PC20 was less than 16 mg/mL. The PC20 was recorded as 100 mg/mL unless the FEV1 decreased by more than 20% after inhalation of the maximum methacholine concentration of 50 mg/mL.
Data are expressed as the mean±standard deviation if they followed a normal distribution and as the median (interquartile range [IQR]) if they showed a skewed distribution. Categorical variables were expressed as counts (percentages). Distributions were examined for normality using the Shapiro-Wilk test. Continuous variables were compared between the BHR-positive and BHR-negative groups using Student's t-test if they were normally distributed and the Mann-Whitney U test otherwise. Categorical variables were compared between the two groups using the chi-square test or Fisher's exact test.
Logistic regression analysis was used to identify predictive factors for positive BHR. Each variable was first analyzed using univariate regression analysis, and variables with a P-value <0.1 were included in the multivariate logistic regression model after excluding multicollinearity. Odds ratio (OR) and 95% confidence interval (CI) were obtained for the selected variables, and the results were adjusted for age and gender.
A receiver operating characteristics (ROC) curve was generated to test the validity of FEF25-75 as a means to distinguish between positive and negative BHR subjects, and the area under the curve (AUC) and its 95% CI were calculated. The cutoff value was obtained from the curve using the Youden method. Patients were subsequently divided into two groups according to the cutoff value and the frequency of BHR was compared between groups with the chi-square test. P-values <0.05 were considered statistically significant, and statistical analyses were done using PASW ver. 18.0 (IBM Co., Armonk, NY, USA).
Among a total of 176 patients, 155 patients (88%) were classified as the BHR-negative group and 21 patients (12%) were classified as the BHR-positive group. Table 1 shows a comparison of demographic characteristics, spirometric parameters, laboratory results and frequency of the number of positive SPT reactions between the two groups. Age, gender, height, and weight did not differ between the two groups. The median PC20 value in the BHR-positive group was 5.74 (IQR, 3.96 to 10.0). FEV1 %predicted, ΔFEV1, FEV1/FVC, ΔFEF25-75 and the other spirometric parameters did not differ between the two groups, whereas FEF25-75 %predicted in the BHR-positive group was significantly lower (P=0.012) compared to the BHR-negative group. Of the laboratory results, both total IgE levels (P<0.0001) and TEC (P=0.002) were significantly higher in the BHR-positive group, as were sIgE levels for D. pteronyssinus (P=0.032) and D. farinae (P=0.011). When the SPT results according to the number of positive reactions (1, 2, 3, 4, and ≥5) were compared, the frequency distribution was not significantly different between the two groups. SPT was performed in 108 patients, all patients with negative SPT results showed a positive reaction in the serological tests.
When analyzed by univariate regression, the variables with a P-value <0.1 were FEF25-75 %predicted (OR, 0.972; 95% CI, 0.949 to 0.996; P=0.023), total IgE (OR, 1.001; 95% CI, 1.000 to 1.001; P=0.044), TEC (OR, 1.001; 95% CI, 1.000 to 1.003; P=0.023), and sIgE levels for D. pteronyssinus (OR, 1.010; 95% CI, 1.000 to 1.019; P=0.049) and D. farinae (OR, 1.011; 95% CI, 1.003 to 1.019; P=0.009). The results of multivariate logistic regression analysis of the variables that were significant on univariate analysis (FEF25-75, total IgE, TEC, and sIgE for D. farinae), adjusting for age and gender, are shown in Table 2. FEF25-75 %predicted (OR, 0.973; 95% CI, 0.948 to 1.000; P=0.046) was the only variable found to be statistically significant.
Fig. 1 shows the ROC curve of FEF25-75 for predicting the presence of BHR. The AUC was 0.669 (95% CI, 0.543 to 0.794; P=0.012) and the optimal cutoff value for distinguishing patients with BHR was 88.4%. Table 3 shows a comparison of the frequency of BHR between the two groups divided based on this cutoff value. A higher frequency of BHR was found in the group with an FEF25-75 less than 88.4% (P<0.0001), and the sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were 57.1%, 80.6%, 28.6%, and 93.3%, respectively.
AR and asthma can be considered one disease taking place in two parts of the respiratory system.29) Airway reversibility to a bronchodilator16) and BHR,14,23) which are paramount features of asthma, are often observed in patients with AR, suggesting a link between AR and asthma. Therefore, this study was performed to obtain data to inform the treatment and monitoring of patients with AR at a higher risk of developing asthma by analyzing the characteristics of children who visited the Pediatric Allergy Clinic and were diagnosed with AR and identifying predictors for the presence of BHR. As a result, 21 of 176 pediatric patients (12%) who were diagnosed as having only AR had BHR. FEF25-75 %predicted, total IgE, TEC, and serum sIgE levels of D. pteronyssinus and D. farinae differed between the BHR-positive and BHR-negative groups, but of these variables, FEF25-75 %predicted was the only statistically significant predictor for the presence of BHR. Unlike a previous study,14) the distributions of the number of SPT-positive reactions did not differ significantly between the two groups and the cutoff value obtained from the ROC curve of FEF25-75 for identifying BHR-positive subjects was 88.4% with a sensitivity of 57.1%, specificity of 80.6%, PPV of 28.6% and NPV of 93.3%.
Even though some studies showed that residual volume/total lung capacity ratio indicating that the degree of hyperinflation reflected the severity of airway obstruction,30) FEF25-75 is thought to correspond to the peripheral airway caliber and has been proposed as a more sensitive parameter than the FEV1 for assessing the presence of a small airway obstruction.31) A low FEF25-75 in the setting of a normal FEV1 is associated with increased asthma severity, systemic steroid use, and asthma exacerbations in children.32) Impaired FEF25-75 has been reported as a marker of early bronchial pathology in patients with AR,9) and studies on the significance of this parameter have also been done in rhinitics. FEF25-75 has been reported to decrease significantly as the duration of AR increases,33) and another study proposed that a greater than 40% decrease in FEF25-75 may be a more useful tool to assess BHR in MCT rather than a 20% decrease in FEV1 in patients with AR.34)
Impaired FEF25-75 less than 65% of the predicted value was a predictive of severe BHR (PC20 <1 mg/mL) in adolescents with AR,35) and the best FEF25-75 cutoff value to distinguish patients with both airway reversibility and severe BHR was reported to be 58.5% in a study of adults patients with AR.25) The value of FEF25-75 with the greatest sensitivity and specificity to detect a 20% increase in FEV1 was 68% in a study conducted in asthmatic children.36) The optimal cutoff value of FEF25-75 to predict the presence of BHR was 88.4% in the present study. This cutoff value is relatively high compared to previous studies and this is thought to be due to the differences in study subjects' characteristics and outcome. In this study, the median age of the BHR-negative group and BHR-positive group was less than 10 and children with short duration AR compared with adults were only included.37) The subjects of this study had relatively good lung function while previous studies examined adults with longer duration AR or asthmatic children. In addition, outcomes of previous studies were severe BHR (PC20 <1 mg/mL)23) or both airway reversibility and severe BHR25) whereas the outcome of this study was BHR (PC20 <16 mg/mL).
In this study, 12% of the total subjects showed positive BHR. Previous studies showed a high frequency of BHR ranging from 39% to 62%,14,19) and in a study of the general population from 8 to 73 years old, BHR-positive rate of 26% was demonstrated.38) The results of the present study is thought to be due to differences in the subjects' characteristics.
One of the limitations of this study is that patients were retrospectively reviewed based on medical records without follow-up. Longitudinal studies are needed to confirm the conclusion that patients with only AR and low FEF25-75 have a higher risk of BHR and they actually are likely to present with asthmatic symptoms in the future. In addition, it will be shown whether the shifting from BHR-negative to BHR-positive group in children with AR can occur through longitudinal studies. Since AR and asthma frequently exist simultaneously,39) it was important to exclude subjects with undetected asthma, which coexists with AR, by examining their medical records thoroughly to screen for symptoms suggestive of asthma. However, the retrospective review was limited. In addition, according to previous studies, patients with perennial rhinitis had a higher risk of BHR than patients with seasonal rhinitis15) and BHR increased two-fold during pollen season in patients with seasonal AR,40) suggesting the type of AR and seasonality could affect BHR. Moreover, the duration of AR can affect the degree of BHR, but these factors were not identified in the patients' medical records and were therefore not considered in the analysis.
In conclusion, the lower airway in patients with AR should be carefully evaluated because they often present with impaired spirometric parameters and BHR. This study emphasizes the role of FEF25-75 in patients suffering from AR, and suggests that reduced FEF25-75 values in children with AR can be helpful in predicting BHR. It is difficult to perform MCT in all children with AR who have only nasal symptoms because they do not show BHR in most cases. Therefore, it seems most cost-effective to perform MCT only in AR patients whose FEF25-75 values are low. Children with low FEF25-75 values on spirometric tests should be followed closely for apparent onset of clinical symptoms of asthma. The results of this study will be helpful in identifying high risk patients with AR who show BHR and treating and monitoring them appropriately. Additional longitudinal studies and intervention studies to assess the incidence of asthma as an outcome will facilitate the understanding of the mechanism underlying these diseases and enable appropriate evaluation and accurate treatment in each patient.
Figures and Tables
 | Fig. 1Receiver operating characteristics curve of forced expiratory flow between 25% and 75% of vital capacity as a diagnostic test for bronchial hyperresponsiveness. The area under the curve was 0.669 (95% confidence interval, 0.543 to 0.794; P=0.012) and the optimal cutoff value to distinguish patients with bronchial hyperresponsiveness was 88.4%. |
Table 1
Patient demographic and clinical characteristics
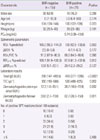
Values are presented as number (%) or median (interquartile range). Continuous variables were compared using the Mann-Whitney U test and categorical variables were compared using the chi-square test or Fisher's exact test.
PC20, provocative concentration causing a 20% decrease in FEV1; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; FEF25-75, forced expiratory flow between 25% and 75% of vital capacity; IgE, immunoglobulin E; TEC, total eosinophil count; SPT, skin prick test.
ACKNOWLEDGMENTS
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2011-0024036 and 2012-0000807).
References
1. Christodoulopoulos P, Cameron L, Durham S, Hamid Q. Molecular pathology of allergic disease. II: Upper airway disease. J Allergy Clin Immunol. 2000. 105(2 Pt 1):211–223.
2. Bousquet J, Bullinger M, Fayol C, Marquis P, Valentin B, Burtin B. Assessment of quality of life in patients with perennial allergic rhinitis with the French version of the SF-36 Health Status Questionnaire. J Allergy Clin Immunol. 1994. 94(2 Pt 1):182–188.

3. Ciprandi G, Cirillo I, Vizzaccaro A, Tosca M, Passalacqua G, Pallestrini E, et al. Seasonal and perennial allergic rhinitis: is this classification adherent to real life? Allergy. 2005. 60:882–887.

4. Bertelsen RJ, Carlsen KC, Carlsen KH. Rhinitis in children: co-morbidities and phenotypes. Pediatr Allergy Immunol. 2010. 21(4 Pt 1):612–622.

5. Asher I. ISAAC International Study of Asthma and Allergies in Childhood. Pediatr Pulmonol. 2007. 42:100.

6. Plaschke PP, Janson C, Norrman E, Bjornsson E, Ellbjar S, Jarvholm B. Onset and remission of allergic rhinitis and asthma and the relationship with atopic sensitization and smoking. Am J Respir Crit Care Med. 2000. 162(3 Pt 1):920–924.

7. Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy. 2008. 63:Suppl 86. 8–160.
8. Suh DI, Lee JK, Lee JH, Koh YY. Bronchodilator response and its relationship to bronchial hyperresponsiveness in children with allergic rhinitis/asthma. Pediatr Allergy Respir Dis. 2010. 20:59–67.
9. Wallace DV, Dykewicz MS, Bernstein DI, Blessing-Moore J, Cox L, Khan DA, et al. The diagnosis and management of rhinitis: an updated practice parameter. J Allergy Clin Immunol. 2008. 122:2 Suppl. S1–S84.

10. Riccio AM, Tosca MA, Cosentino C, Pallestrini E, Ameli F, Canonica GW, et al. Cytokine pattern in allergic and non-allergic chronic rhinosinusitis in asthmatic children. Clin Exp Allergy. 2002. 32:422–426.

11. Ciprandi G, Cirillo I, Vizzaccaro A, Milanese M, Tosca MA. Airway function and nasal inflammation in seasonal allergic rhinitis and asthma. Clin Exp Allergy. 2004. 34:891–896.

12. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease (COPD) and asthma. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, November 1986. Am Rev Respir Dis. 1987. 136:225–244.
13. Jeffery PK, Wardlaw AJ, Nelson FC, Collins JV, Kay AB. Bronchial biopsies in asthma. An ultrastructural, quantitative study and correlation with hyperreactivity. Am Rev Respir Dis. 1989. 140:1745–1753.

14. Mete N, Sin A, Gulbahar O, Erdinc M, Sebik F, Kokuludag A. The determinants of bronchial hyperresponsiveness in patients with allergic rhinitis. Ann Allergy Asthma Immunol. 2004. 93:193–199.

15. Choi SH, Yoo Y, Yu J, Rhee CS, Min YG, Koh YY. Bronchial hyperresponsiveness in young children with allergic rhinitis and its risk factors. Allergy. 2007. 62:1051–1056.

16. Suh DI, Lee JK, Kim JT, Min YG, Koh YY. Bronchial hyperresponsiveness in preschool children with allergic rhinitis. Am J Rhinol Allergy. 2011. 25:e186–e190.

17. Ramsdale EH, Morris MM, Roberts RS, Hargreave FE. Asymptomatic bronchial hyperresponsiveness in rhinitis. J Allergy Clin Immunol. 1985. 75:573–577.

18. Braman SS, Barrows AA, DeCotiis BA, Settipane GA, Corrao WM. Airway hyperresponsiveness in allergic rhinitis. A risk factor for asthma. Chest. 1987. 91:671–674.
19. Jang AS. Nasal eosinophilic inflammation contributes to bronchial hyperresponsiveness in patients with allergic rhinitis. J Korean Med Sci. 2002. 17:761–764.

20. Ciprandi G, Tosca MA, Cirillo I, Capasso M. Impact of allergic rhinitis on asthma in children: effects on bronchial hyperreactivity. Allergy. 2010. 65:1199–1201.

21. Cuttitta G, Cibella F, La Grutta S, Hopps MR, Bucchieri S, Passalacqua G, et al. Non-specific bronchial hyper-responsiveness in children with allergic rhinitis: relationship with the atopic status. Pediatr Allergy Immunol. 2003. 14:458–463.

22. Foresi A, Leone C, Pelucchi A, Mastropasqua B, Chetta A, D'Ippolito R, et al. Eosinophils, mast cells, and basophils in induced sputum from patients with seasonal allergic rhinitis and perennial asthma: relationship to methacholine responsiveness. J Allergy Clin Immunol. 1997. 100:58–64.

23. Ciprandi G, Tosca MA, Signori A, Cirillo I. Bronchial hyperreactivity in patients with allergic rhinitis: forced expiratory flow between 25 and 75% of vital capacity might be a predictive factor. Allergy Asthma Proc. 2011. 32:4–8.

24. Parker AL, Abu-Hijleh M, McCool FD. Ratio between forced expiratory flow between 25% and 75% of vital capacity and FVC is a determinant of airway reactivity and sensitivity to methacholine. Chest. 2003. 124:63–69.

25. Ciprandi G, Signori A, Cirillo I. Relationship between bronchial hyperreactivity and bronchodilation in patients with allergic rhinitis. Ann Allergy Asthma Immunol. 2011. 106:460–466.

26. Koh YY, Lee MH, Kim CK, Min YG, Kim YK, Min KU, et al. A familial predisposition in bronchial hyperresponsiveness among patients with allergic rhinitis. J Allergy Clin Immunol. 1998. 102(6 Pt 1):921–926.

27. American Thoracic Society. Standardization of Spirometry, 1994 Update. Am J Respir Crit Care Med. 1995. 152:1107–1136.
28. Takase M, Sakata H, Shikada M, Tatara K, Fukushima T, Miyakawa T. Development of reference equations for spirometry in Japanese children aged 6-18 years. Pediatr Pulmonol. 2013. 48:35–44.

29. Blaiss MS. Rhinitis-asthma connection: epidemiologic and pathophysiologic basis. Allergy Asthma Proc. 2005. 26:35–40.
30. Mattiello R, Mallol J, Fischer GB, Mocelin HT, Rueda B, Sarria EE. Pulmonary function in children and adolescents with postinfectious bronchiolitis obliterans. J Bras Pneumol. 2010. 36:453–459.
31. Bjermer L. History and future perspectives of treating asthma as a systemic and small airways disease. Respir Med. 2001. 95:703–719.

32. Rao DR, Gaffin JM, Baxi SN, Sheehan WJ, Hoffman EB, Phipatanakul W. The utility of forced expiratory flow between 25% and 75% of vital capacity in predicting childhood asthma morbidity and severity. J Asthma. 2012. 49:586–592.

33. Ciprandi G, Cirillo I, Pistorio A. Impact of allergic rhinitis on asthma: effects on spirometric parameters. Allergy. 2008. 63:255–260.

34. Munoz-Lopez F, Rios-Alcolea M. The interest of FEF(25-75) in evaluating bronchial hyperresponsiveness with the methacholine test. Allergol Immunopathol (Madr). 2012. 40:352–356.

35. Ciprandi G, Tosca MA, Castellazzi AM, Cairello F, Salpietro C, Arrigo T, et al. FEF(25-75) might be a predictive factor for bronchial inflammation and bronchial hyperreactivity in adolescents with allergic rhinitis. Int J Immunopathol Pharmacol. 2011. 24:4 Suppl. 17–20.

36. Simon MR, Chinchilli VM, Phillips BR, Sorkness CA, Lemanske RF Jr, Szefler SJ, et al. Forced expiratory flow between 25% and 75% of vital capacity and FEV1/forced vital capacity ratio in relation to clinical and physiological parameters in asthmatic children with normal FEV1 values. J Allergy Clin Immunol. 2010. 126:527.e1–534.e8.

37. Skoner DP. Allergic rhinitis: definition, epidemiology, pathophysiology, detection, and diagnosis. J Allergy Clin Immunol. 2001. 108:1 Suppl. S2–S8.

38. Paoletti P, Carrozzi L, Viegi G, Modena P, Ballerin L, Di Pede F, et al. Distribution of bronchial responsiveness in a general population: effect of sex, age, smoking, and level of pulmonary function. Am J Respir Crit Care Med. 1995. 151:1770–1777.

39. Simons FE. Allergic rhinobronchitis: the asthma-allergic rhinitis link. J Allergy Clin Immunol. 1999. 104(3 Pt 1):534–540.

40. Di Lorenzo G, Mansueto P, Melluso M, Morici G, Norrito F, Esposito Pellitteri M, et al. Non-specific airway hyperresponsiveness in mono-sensitive Sicilian patients with allergic rhinitis. Its relationship to total serum IgE levels and blood eosinophils during and out of the pollen season. Clin Exp Allergy. 1997. 27:1052–1059.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download