Abstract
For the past two decades, a huge number of genetic studies have been conducted to identify the genetic variants responsible for asthma risk. Several types of genetic and genomic approaches, including linkage analysis, candidate gene single nucleotide poly-morphism studies, and whole genome-wide association studies have been applied. However, the genetic impacts of these studies are minimal because asthma is a complex syndrome affected by interaction with many environmental factors mediated by epigenetics. Epigenetics is alteration of genetic expression without changes of DNA sequence. Three major forms of epigenetic is DNA methylation, histone modfications and specific microRNA expression that are known to have vast effects on gene expression. How-ever, knowledge regarding the epigenetic effect on the development of asthma and its traits is limited up to date. Recently, new data on epigenetics have been brought up to explain the phenotypic alterations of asthma. In this review, we present general con-cept of epigenetics, environmental factors inducting epigenetic changes and the background mechanisms in epigenetics behind development asthma and epigenetic therapy.
REFERENCES
2. Cortessis VK, Thomas DC, Levine AJ, Breton CV, Mack TM, Siegmund KD, et al. Environmental epigenetics: prospects for studying epigenetic mediation of exposure-response relationships. Hum Genet. 2012; 131:1565–89.

4. Turker MS, Bestor TH. Formation of methylation patterns in the mam-malian genome. Mutat Res. 1997; 386:119–30.

5. Okitsu CY, Hsieh CL. DNA methylation dictates histone H3K4 methylation. Mol Cell Biol. 2007; 27:2746–57.

7. Kitamoto S, Yamada N, Yokoyama S, Houjou I, Higashi M, Goto M, et al. DNA methylation and histone H3-K9 modifications contribute to MUC17 expression. Glycobiology. 2011; 21:247–56.

8. Stewart MD, Li J, Wong J. Relationship between histone H3 lysine 9 methylation, transcription repression, and heterochromatin protein 1 re-cruitment. Mol Cell Biol. 2005; 25:2525–38.

9. Cedar H, Bergman Y. Linking DNA methylation and histone modification: patterns and paradigms. Nat Rev Genet. 2009; 10:295–304.

11. Chen K, Rajewsky N. The evolution of gene regulation by transcription factors and microRNAs. Nat Rev Genet. 2007; 8:93–103.

13. Miller RL, Ho SM. Environmental epigenetics and asthma: current concepts and call for studies. Am J Respir Crit Care Med. 2008; 177:567–73.
14. Gilliland FD, Berhane K, Li YF, Rappaport EB, Peters JM. Effects of early onset asthma and in utero exposure to maternal smoking on childhood lung function. Am J Respir Crit Care Med. 2003; 167:917–24.
15. Li YF, Langholz B, Salam MT, Gilliland FD. Maternal and grandmaternal smoking patterns are associated with early childhood asthma. Chest. 2005; 127:1232–41.

16. Magnusson LL, Olesen AB, Wennborg H, Olsen J. Wheezing, asthma, hayfever, and atopic eczema in childhood following exposure to tobacco smoke in fetal life. Clin Exp Allergy. 2005; 35:1550–6.

17. Alati R, Al Mamun A, O'Callaghan M, Najman JM, Williams GM. In utero and postnatal maternal smoking and asthma in adolescence. Epidemiology. 2006; 17:138–44.

18. Kurukulaaratchy RJ, Matthews S, Arshad SH. Does environment mediate earlier onset of the persistent childhood asthma phenotype? Pediatrics. 2004; 113:345–50.

19. Miller RL, Garfinkel R, Horton M, Camann D, Perera FP, Whyatt RM, et al. Polycyclic aromatic hydrocarbons, environmental tobacco smoke, and respiratory symptoms in an inner-city birth cohort. Chest. 2004; 126:1071–8.

20. Enstrom JE, Kabat GC. Environmental tobacco smoke and tobacco related mortality in a prospective study of Californians, 1960-98. BMJ. 2003; 326:1057.

21. Dolinoy DC, Weidman JR, Jirtle RL. Epigenetic gene regulation: linking early developmental environment to adult disease. Reprod Toxicol. 2007; 23:297–307.

22. Tang WY, Ho SM. Epigenetic reprogramming and imprinting in origins of disease. Rev Endocr Metab Disord. 2007; 8:173–82.

23. Devereux G, Turner SW, Craig LC, McNeill G, Martindale S, Harbour PJ, et al. Low maternal vitamin E intake during pregnancy is associated with asthma in 5-year-old children. Am J Respir Crit Care Med. 2006; 174:499–507.

24. Jedrychowski W, Gałas A, Whyatt R, Perera F. The prenatal use of antibiotics and the development of allergic disease in one year old infants. A preliminary study. Int J Occup Med Environ Health. 2006; 19:70–6.

25. Kukkonen K, Savilahti E, Haahtela T, Juntunen-Backman K, Korpela R, Poussa T, et al. Probiotics and prebiotic galacto-oligosaccharides in the prevention of allergic diseases: a randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2007; 119:192–8.

26. Fitzsimon N, Fallon U, O'Mahony D, Loftus BG, Bury G, Murphy AW, et al. Mothers' dietary patterns during pregnancy and risk of asthma symptoms in children at 3 years. Ir Med J. 2007; 100(suppl):27–32.
27. Hamada K, Suzaki Y, Leme A, Ito T, Miyamoto K, Kobzik L, et al. Exposure of pregnant mice to an air pollutant aerosol increases asthma susceptibility in offspring. J Toxicol Environ Health A. 2007; 70:688–95.

28. Fedulov AV, Leme A, Yang Z, Dahl M, Lim R, Mariani TJ, et al. Pulmo-nary exposure to particles during pregnancy causes increased neonatal asthma susceptibility. Am J Respir Cell Mol Biol. 2008; 38:57–67.

29. Blümer N, Herz U, Wegmann M, Renz H. Prenatal lipopolysaccharide-exposure prevents allergic sensitization and airway inflammation, but not airway responsiveness in a murine model of experimental asthma. Clin Exp Allergy. 2005; 35:397–402.

30. Blumer N, Sel S, Virna S, Patrascan CC, Zimmermann S, Herz U, et al. Perinatal maternal application of Lactobacillus rhamnosus GG suppresses allergic airway inflammation in mouse offspring. Clin Exp Allergy. 2007; 37:348–57.

31. Rahman I. Oxidative stress, chromatin remodeling and gene transcription in inflammation and chronic lung diseases. J Biochem Mol Biol. 2003; 36:95–109.

32. Rahman I, Marwick J, Kirkham P. Redox modulation of chromatin remodeling: impact on histone acetylation and deacetylation, NF-kappaB and pro-inflammatory gene expression. Biochem Pharmacol. 2004; 68:1255–67.
33. Ito K, Caramori G, Lim S, Oates T, Chung KF, Barnes PJ, et al. Expression and activity of histone deacetylases in human asthmatic airways. Am J Respir Crit Care Med. 2002; 166:392–6.

34. Gilliland FD, Berhane K, McConnell R, Gauderman WJ, Vora H, Rappaport EB, et al. Maternal smoking during pregnancy, environmental tobacco smoke exposure and childhood lung function. Thorax. 2000; 55:271–6.

35. Bush A, Menzies-Gow A. Phenotypic differences between pediatric and adult asthma. Proc Am Thorac Soc. 2009; 6:712–9.

36. Mandhane PJ, Greene JM, Cowan JO, Taylor DR, Sears MR. Sex differences in factors associated with childhood- and adolescent-onset wheeze. Am J Respir Crit Care Med. 2005; 172:45–54.

37. Perera FP, Rauh V, Tsai WY, Kinney P, Camann D, Barr D, et al. Effects of transplacental exposure to environmental pollutants on birth outcomes in a multiethnic population. Environ Health Perspect. 2003; 111:201–5.

38. Windham GC, Hopkins B, Fenster L, Swan SH. Prenatal active or passive tobacco smoke exposure and the risk of preterm delivery or low birth weight. Epidemiology. 2000; 11:427–33.

39. Su RC, Becker AB, Kozyrskyj AL, Hayglass KT. Altered epigenetic regulation and increasing severity of bronchial hyperresponsiveness in atopic asthmatic children. J Allergy Clin Immunol. 2009; 124:1116–8.

40. Nadeau K, McDonald-Hyman C, Noth EM, Pratt B, Hammond SK, Balmes J, et al. Ambient air pollution impairs regulatory T-cell function in asthma. J Allergy Clin Immunol. 2010; 126:845–852.e10.

41. Cheong HS, Park SM, Kim MO, Park JS, Lee JY, Byun JY, et al. Genome-wide methylation profile of nasal polyps: relation to aspirin hypersensitivity in asthmatics. Allergy. 2011; 66:637–44.

42. Lee JS, Kim JH, Bae JS, Kim JY, Park TJ, Pasaje CF, et al. Association of CACNG6 polymorphisms with aspirin-intolerance asthmatics in a Korean population. BMC Med Genet. 2010; 11:138.

43. Yoo CB, Jones PA. Epigenetic therapy of cancer: past, present and future. Nat Rev Drug Discov. 2006; 5:37–50.

44. Juergens RA, Wrangle J, Vendetti FP, Murphy SC, Zhao M, Coleman B, et al. Combination epigenetic therapy has efficacy in patients with refractory advanced non-small cell lung cancer. Cancer Discov. 2011; 1:598–607.

45. Egger G, Liang G, Aparicio A, Jones PA. Epigenetics in human disease and prospects for epigenetic therapy. Nature. 2004; 429:457–63.

46. Chen M, Shabashvili D, Nawab A, Yang SX, Dyer LM, Brown KD, et al. DNA methyltransferase inhibitor, zebularine, delays tumor growth and induces apoptosis in a genetically engineered mouse model of breast cancer. Mol Cancer Ther. 2012; 11:370–82.

47. Billam M, Sobolewski MD, Davidson NE. Effects of a novel DNA methyltransferase inhibitor zebularine on human breast cancer cells. Breast Cancer Res Treat. 2010; 120:581–92.

48. Breton CV, Byun HM, Wenten M, Pan F, Yang A, Gilliland FD. Prenatal tobacco smoke exposure affects global and gene-specific DNA methylation. Am J Respir Crit Care Med. 2009; 180:462–7.

49. Ito K, Lim S, Caramori G, Chung KF, Barnes PJ, Adcock IM. Cigarette smoking reduces histone deacetylase 2 expression, enhances cytokine expression, and inhibits glucocorticoid actions in alveolar macrophages. FASEB J. 2001; 15:1110–2.

50. Launay JM, Del Pino M, Chironi G, Callebert J, Peoc'h K, Megnien JL, et al. Smoking induces long-lasting effects through a monoamine-oxidase epigenetic regulation. PLoS One. 2009; 4:e7959.

51. Perera F, Tang WY, Herbstman J, Tang D, Levin L, Miller R, et al. Relation of DNA methylation of 5'-CpG island of ACSL3 to transplacental exposure to airborne polycyclic aromatic hydrocarbons and childhood asthma. PLoS One. 2009; 4:e4488.

52. Tang WY, Levin L, Talaska G, Cheung YY, Herbstman J, Tang D, et al. Maternal exposure to polycyclic aromatic hydrocarbons and 5'-CpG methylation of interferon-γ in cord white blood cells. Environ Health Perspect. 2012; 120:1195–200.

53. Kwon NH, Kim JS, Lee JY, Oh MJ, Choi DC. DNA methylation and the expression of IL-4 and IFN-gamma promoter genes in patients with bronchial asthma. J Clin Immunol. 2008; 28:139–46.
54. Breton CV, Byun HM, Wang X, Salam MT, Siegmund K, Gilliland FD. DNA methylation in the arginase-nitric oxide synthase pathway is associated with exhaled nitric oxide in children with asthma. Am J Respir Crit Care Med. 2011; 184:191–7.

55. Wu W, Doreswamy V, Diaz-Sanchez D, Samet JM, Kesic M, Dailey L, et al. GSTM1 modulation of IL-8 expression in human bronchial epithelial cells exposed to ozone. Free Radic Biol Med. 2011; 51:522–9.

56. Chiba T, Marusawa H, Ushijima T. Inflammation-associated cancer development in digestive organs: mechanisms and roles for genetic and epigenetic modulation. Gastroenterology. 2012; 143:550–63.

Fig. 2.
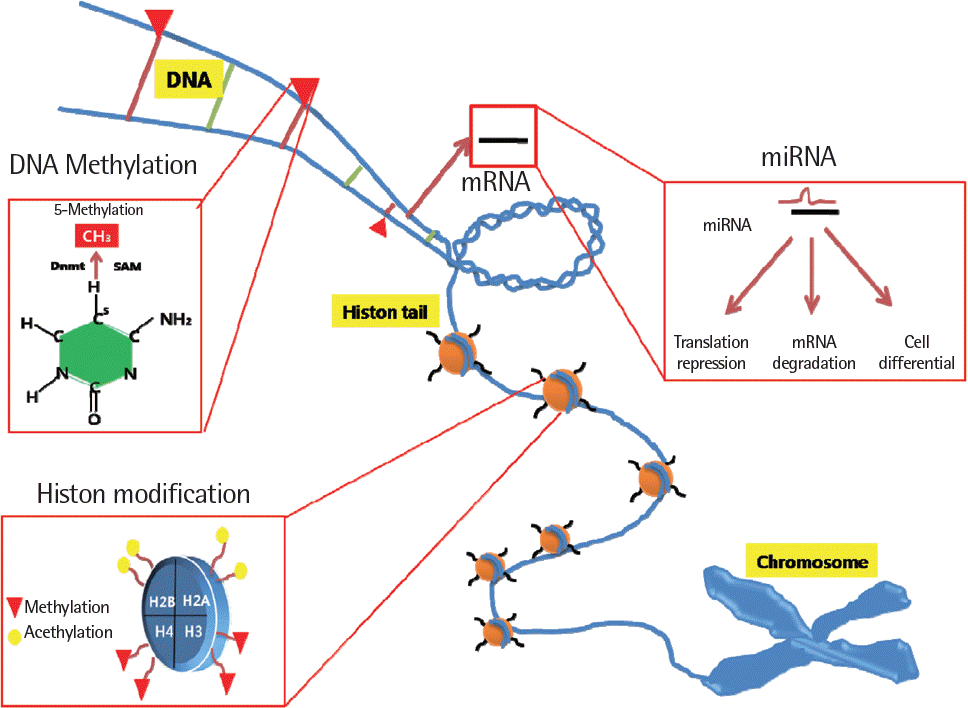
DNA metylation, chromatin remodeling of the gene expression. (A) The DNA methylation machinery interacts with the histone modification machinery. (B) DN-MTs, MeCP2, HDAC3 and MBPs recognized by other transcriptional regulators. TF, transcription Factor; PolII, DNA polymerase II; HAT, histone acetyltransferase; MBP, methyl-bindg protein; MeCP2, methyl CpG binding protein; DNMT, DNA methyl transferase; HDAC, histone deacetylases.
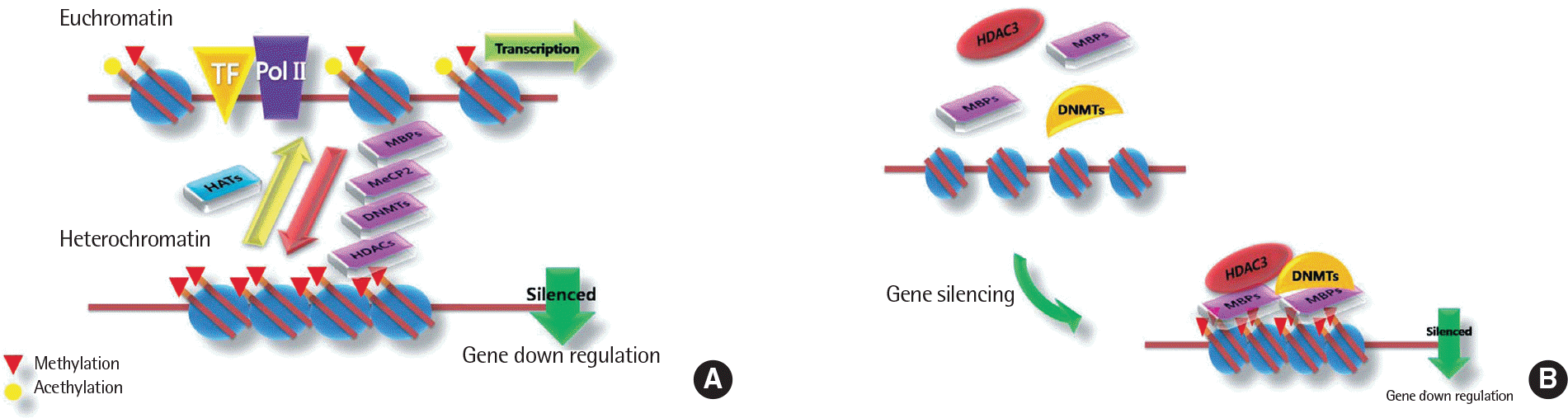
Fig. 3.
Nucleosome structure and histone acetylation of a lysine. (A) Histones H2A, H2B, H3 and H4 are known as the core histones, this nucleosome has six N-termi-nal tail domains and two C-terminal tails. (B) Histone acetyltransferases histone acetyltransferases and histone deacetylases histone deacetylases recognized by other transcriptional regulators. K, lysine; P, phosphate; Ub, ubiquitin; S, serine; E, glutamic acid; C, carboxyl terminus; N, amino terminus; H, histone.
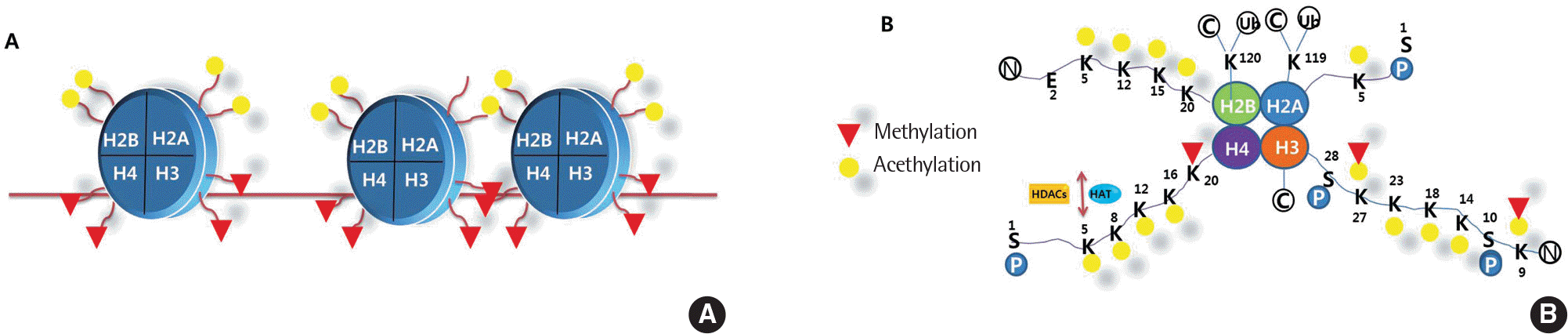
Fig. 4.
Odd ratio of single nucleotide mutations and genes associated with asthma and asthma phenotypes (2003–2010, Soonchunhyang Genome Research Center). Single base mutations of the genes involved in innate immune process-es and acquired immunity, of asthma-related risk. IL, interleukin; TLR, toll-like receptors; CD4, cluster of differentiation 4; FcRI, high-affinity immunoglobulin E receptor; NFAT, nuclear factor of activated T-cells; API, arrowhead proteinase in-hibitor; GATA, globin transcription factor; NFkB, nuclear factor of kappa light polypeptide gene enhancer in B-cells 1; CXCR, C-X-C chemokine receptor type; CTNNA, catenin; CSF1R, colony stimulating factor 1 receptor; PPAR, peroxisome proliferator-activated receptor; MCP3, chemokine (C-C motif) ligand; DCNP1, chromosome 5 open reading frame; RUX1, runt-related transcription factor 1; ITK, IL 2-inducible T-cell kinase; STAT, signal transducer and activator of transcription; ADAM, metallopeptidase domain; MYLK, myosin light chain kinase.
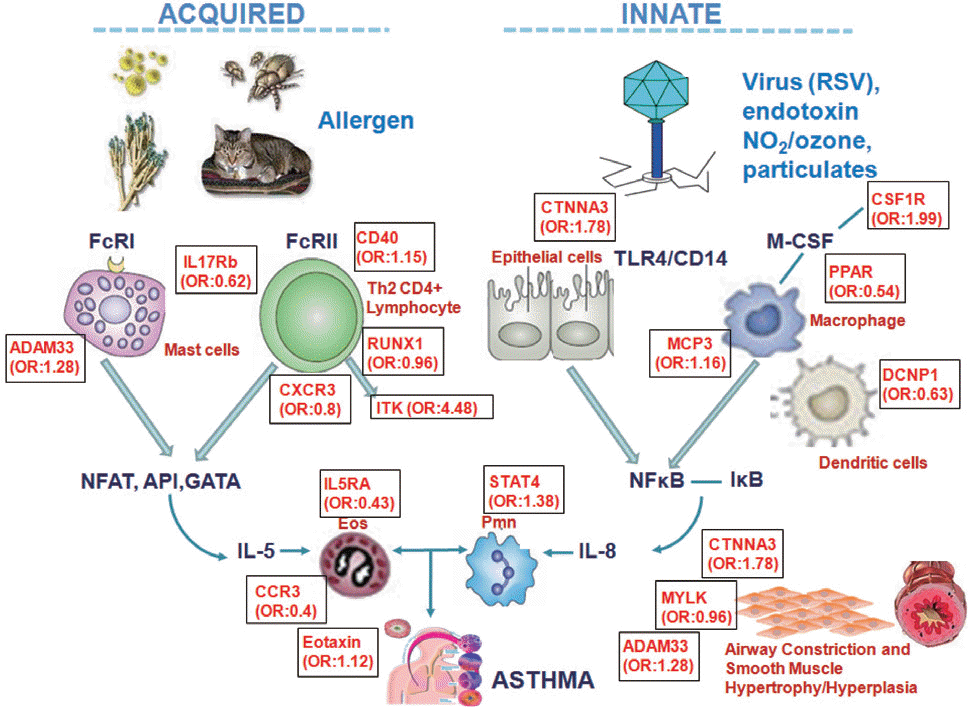
Fig. 5.
Factors associated with the type of inflammatory cells to determine the diversity of the asthma phenotype. BaP, benzophenone; PAH, polycyclic aromatic hydrocarbons; DEP, diesel exhaust particles; PM, particulate matter; LPS, lipo-polysaccharide; IL, interleukin; TH cell, T helper cell; IFN, interferon; TGF-β, transforming growth factor beta; GATA, globin transcription factor; FOXP3, fork-head box P3; T-bet, T-box transcription factor.
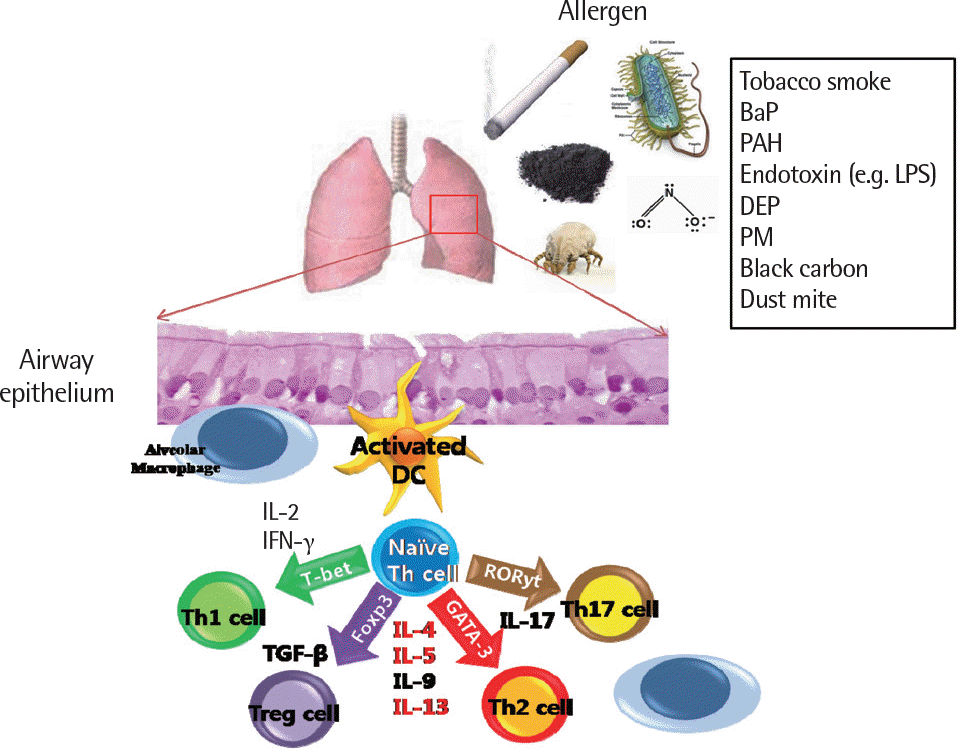
Table 1.
Environmental factors associated with epigenetics
GSTP1, glutathione S-transferase pi gene; GSTM1, glutathione S-transferase Mu 1; HDAC, histone deacetylase; MABO, monoamine oxidase B; IFN, interferon; PBMC, peripheral blood mononuclear cell; PAH, polycyclic aromatic hydrocarbons; UCWBC, umbilical cord white blood cell; ARG, arginase; HAT, histone acetyltransferase.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download